BK channel density is regulated by endoplasmic reticulum associated degradation and influenced by the SKN-1A/NRF1 transcription factor
- PMID: 32502151
- PMCID: PMC7299407
- DOI: 10.1371/journal.pgen.1008829
BK channel density is regulated by endoplasmic reticulum associated degradation and influenced by the SKN-1A/NRF1 transcription factor
Abstract
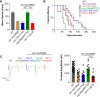
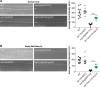
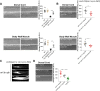
Ion channels are present at specific levels within subcellular compartments of excitable cells. The regulation of ion channel trafficking and targeting is an effective way to control cell excitability. The BK channel is a calcium-activated potassium channel that serves as a negative feedback mechanism at presynaptic axon terminals and sites of muscle excitation. The C. elegans BK channel ortholog, SLO-1, requires an endoplasmic reticulum (ER) membrane protein for efficient anterograde transport to these locations. Here, we found that, in the absence of this ER membrane protein, SLO-1 channels that are seemingly normally folded and expressed at physiological levels undergo SEL-11/HRD1-mediated ER-associated degradation (ERAD). This SLO-1 degradation is also indirectly regulated by a SKN-1A/NRF1-mediated transcriptional mechanism that controls proteasome levels. Therefore, our data indicate that SLO-1 channel density is regulated by the competitive balance between the efficiency of ER trafficking machinery and the capacity of ERAD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10: 3664–84. Available: http://www.ncbi.nlm.nih.gov/pubmed/1700083 10.1523/JNEUROSCI.10-11-03664.1990 - DOI - PMC - PubMed
-
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21: 9585–9597. 21/24/9585 [pii] 10.1523/JNEUROSCI.21-24-09585.2001 - DOI - PMC - PubMed
-
- Knot H. Ryanodine receptors regulate arterial wall and diameter in cerebral arteries of rat via K Ca channels. J Physiol. 1998; 211–221. Available: http://scholar.google.co.uk/scholar?hl=en&q=%22hj+knot%22&as_sdt=0,5&as_... - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

