Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--The BRACE CORONA Trial
- PMID: 32502882
- PMCID: PMC7219415
- DOI: 10.1016/j.ahj.2020.05.002
Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--The BRACE CORONA Trial
Abstract
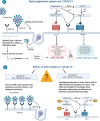
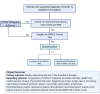
Angiotensin-converting enzyme-2 (ACE2) expression may increase due to upregulation in patients using angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs). Because renin-angiotensin system blockers increase levels of ACE2, a protein that facilitates coronavirus entry into cells, there is concern that these drugs could increase the risk of developing a severe and fatal form of COVID-19. The impact of discontinuing ACEI and ARBs in patients with COVID-19 remains uncertain. DESIGN: BRACE CORONA is a pragmatic, multicenter, randomized, phase IV, clinical trial that aims to enroll around 500 participants at 34 sites in Brazil. Participants will be identified from an ongoing national registry of suspected and confirmed cases of COVID-19. Eligible patients using renin-angiotensin system blockers (ACEI/ARBs) with a confirmed diagnosis of COVID-19 will be randomized to a strategy of continued ACEI/ARB treatment versus temporary discontinuation for 30 days. The primary outcome is the median days alive and out of the hospital at 30 days. Secondary outcomes include progression of COVID-19 disease, all-cause mortality, death from cardiovascular causes, myocardial infarction, stroke, transient ischemic attack, new or worsening heart failure, myocarditis, pericarditis, arrhythmias, thromboembolic events, hypertensive crisis, respiratory failure, hemodynamic decompensation, sepsis, renal failure, and troponin, B-type natriuretic peptide (BNP), N-terminal-proBNP, and D-dimer levels. SUMMARY: BRACE CORONA will evaluate whether the strategy of continued ACEI/ARB therapy compared with temporary discontinuation of these drugs impacts clinical outcomes among patients with COVID-19.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures


References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19): situation report, 51. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Published: March 11, 2020. Accessed April 24, 2020.
-
- Bothwell L.E., Podolsky S.H. The emergence of the randomized, controlled trial. N Engl J Med. 2016;375:501–504. - PubMed
-
- Fanaroff A.C., Califf R.M., Lopes R.D. New approaches to conducting randomized controlled trials. J Am Coll Cardiol. 2020;75:556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

