Nanotechnology as a Platform for the Development of Injectable Parenteral Formulations: A Comprehensive Review of the Know-Hows and State of the Art
- PMID: 32503171
- PMCID: PMC7356945
- DOI: 10.3390/pharmaceutics12060510
Nanotechnology as a Platform for the Development of Injectable Parenteral Formulations: A Comprehensive Review of the Know-Hows and State of the Art
Abstract
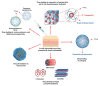
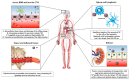
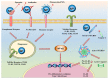
Within recent decades, the development of nanotechnology has made a significant contribution to the progress of various fields of study, including the domains of medical and pharmaceutical sciences. A substantially transformed arena within the context of the latter is the development and production of various injectable parenteral formulations. Indeed, recent decades have witnessed a rapid growth of the marketed and pipeline nanotechnology-based injectable products, which is a testimony to the remarkability of the aforementioned contribution. Adjunct to the ability of nanomaterials to deliver the incorporated payloads to many different targets of interest, nanotechnology has substantially assisted to the development of many further facets of the art. Such contributions include the enhancement of the drug solubility, development of long-acting locally and systemically injectable formulations, tuning the onset of the drug's release through the endowment of sensitivity to various internal or external stimuli, as well as adjuvancy and immune activation, which is a desirable component for injectable vaccines and immunotherapeutic formulations. The current work seeks to provide a comprehensive review of all the abovementioned contributions, along with the most recent advances made within each domain. Furthermore, recent developments within the domains of passive and active targeting will be briefly debated.
Keywords: adjuvancy; controlled release; immune activation; injectable parenteral formulations; nanotechnology; solubility enhancement; targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Vega J.A., Ochoa P.S., Holder P. Concepts in Steril Preparations and Aseptic Techniques. Jones and Bartlett Learning; Burlington, MA, USA: 2015. Introduction to parenteral preparations; pp. 1–26.
-
- Patravale V., Prajakta D., Jain R. Nanoparticulate Drug Delivery: Perspectives on the Transition from Laboratory to Market. Woodhead Publishing; New Dehli, India: 2012. Nanoparticles as drug carriers; pp. 29–86.
Publication types
LinkOut - more resources
Full Text Sources

