Studies on the Properties of the Sporulation Specific Protein Dit1 and its Product Formyl Tyrosine
- PMID: 32503197
- PMCID: PMC7345447
- DOI: 10.3390/jof6020077
Studies on the Properties of the Sporulation Specific Protein Dit1 and its Product Formyl Tyrosine
Abstract
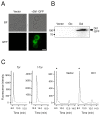
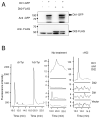
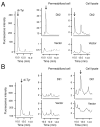
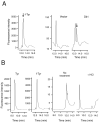
The dityrosine layer is a unique structure present in the spore wall of the budding yeast Saccharomyces cerevisiae. The primary constituent of this layer is bisformyl dityrosine. A sporulation-specific protein, Dit1 is localized in the spore cytosol and produces a precursor of bisformyl dityrosine. Although Dit1 is similar to isocyanide synthases, the loss of Dit1 is not rescued by heterologous expression of the Pseudomonas aeruginosa isocyanide synthase, PvcA, indicating that Dit1 does not mediate isocyanidation. The product of Dit1 is most likely formyl tyrosine. Dit1 can produce its product when it is expressed in vegetative cells; however, formyl tyrosine was not detected in the crude cell lysate. We reasoned that formyl tyrosine is unstable and reacts with some molecule to form formyl tyrosine-containing molecules in the cell lysate. In support of this hypothesis, formyl tyrosine was detected when the lysate was hydrolyzed with a mild acid. The same property was also found for bisformyl dityrosine. Bisformyl dityrosine molecules assemble to form the dityrosine layer by an unknown mechanism. Given that bisformyl dityrosine can be released from the spore wall by mild hydrolysis, the process of formyl tyrosine-containing molecule formation may resemble the assembly of the dityrosine layer.
Keywords: Dit1; Saccharomyces cerevisiae; dityrosine layer; formyl tyrosine; spore wall.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Characterization of a yeast sporulation-specific P450 family protein, Dit2, using an in vitro assay to crosslink formyl tyrosine.J Biochem. 2018 Feb 1;163(2):123-131. doi: 10.1093/jb/mvx067. J Biochem. 2018. PMID: 29365103
-
In vitro reconstitution of the yeast spore wall dityrosine layer discloses the mechanism of its assembly.J Biol Chem. 2017 Sep 22;292(38):15880-15891. doi: 10.1074/jbc.M117.786202. Epub 2017 Aug 9. J Biol Chem. 2017. PMID: 28794156 Free PMC article.
-
The sporulation-specific enzymes encoded by the DIT1 and DIT2 genes catalyze a two-step reaction leading to a soluble LL-dityrosine-containing precursor of the yeast spore wall.Proc Natl Acad Sci U S A. 1994 May 10;91(10):4524-8. doi: 10.1073/pnas.91.10.4524. Proc Natl Acad Sci U S A. 1994. PMID: 8183942 Free PMC article.
-
Dityrosine as a product of oxidative stress and fluorescent probe.Amino Acids. 2003 Dec;25(3-4):233-47. doi: 10.1007/s00726-003-0014-z. Epub 2003 Aug 21. Amino Acids. 2003. PMID: 14661087 Review.
-
Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins.Toxicology. 2002 Aug 1;177(1):11-22. doi: 10.1016/s0300-483x(02)00192-0. Toxicology. 2002. PMID: 12126792 Review.
Cited by
-
Mining for a new class of fungal natural products: the evolution, diversity, and distribution of isocyanide synthase biosynthetic gene clusters.Nucleic Acids Res. 2023 Aug 11;51(14):7220-7235. doi: 10.1093/nar/gkad573. Nucleic Acids Res. 2023. PMID: 37427794 Free PMC article.
-
Improved production of Taxol® precursors in S. cerevisiae using combinatorial in silico design and metabolic engineering.Microb Cell Fact. 2023 Nov 29;22(1):243. doi: 10.1186/s12934-023-02251-7. Microb Cell Fact. 2023. PMID: 38031061 Free PMC article.
-
Special Issue: Formation and Function of Fungal Ascospores.J Fungi (Basel). 2021 Jul 29;7(8):618. doi: 10.3390/jof7080618. J Fungi (Basel). 2021. PMID: 34436157 Free PMC article.
-
Mining for a New Class of Fungal Natural Products: The Evolution, Diversity, and Distribution of Isocyanide Synthase Biosynthetic Gene Clusters.bioRxiv [Preprint]. 2023 Apr 18:2023.04.17.537281. doi: 10.1101/2023.04.17.537281. bioRxiv. 2023. Update in: Nucleic Acids Res. 2023 Aug 11;51(14):7220-7235. doi: 10.1093/nar/gkad573. PMID: 37131656 Free PMC article. Updated. Preprint.
References
Grants and funding
- JUSRP51629B/Fundamental Research Funds for the Central Universities
- Program of Introducing Talents of Discipline to Universities/Program of Introducing Talents of Discipline to Universities
- LITE2018-015/National first-class discipline program of Light Industry Technology and Engineering
- 21576118/National Natural Science Foundation of China
- 21778023/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

