Influence of Calcium Binding on Conformations and Motions of Anionic Polyamino Acids. Effect of Side Chain Length
- PMID: 32503199
- PMCID: PMC7362111
- DOI: 10.3390/polym12061279
Influence of Calcium Binding on Conformations and Motions of Anionic Polyamino Acids. Effect of Side Chain Length
Abstract
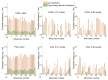
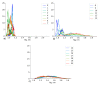
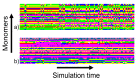
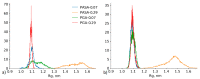
Investigation of the effect of CaCl2 salt on conformations of two anionic poly(amino acids) with different side chain lengths, poly-(α-l glutamic acid) (PGA) and poly-(α-l aspartic acid) (PASA), was performed by atomistic molecular dynamics (MD) simulations. The simulations were performed using both unbiased MD and the Hamiltonian replica exchange (HRE) method. The results show that at low CaCl2 concentration adsorption of Ca2+ ions lead to a significant chain size reduction for both PGA and PASA. With the increase in concentration, the chains sizes partially recover due to electrostatic repulsion between the adsorbed Ca2+ ions. Here, the side chain length becomes important. Due to the longer side chain and its ability to distance the charged groups with adsorbed ions from both each other and the backbone, PGA remains longer in the collapsed state as the CaCl2 concentration is increased. The analysis of the distribution of the mineral ions suggests that both poly(amino acids) should induce the formation of mineral with the same structure of the crystal cell.
Keywords: Hamiltonian replica exchange; mineralization; molecular dynamic simulation; poly(amino acids); poly-(α-l aspartic acid); poly-(α-l glutamic acid); salt solutions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




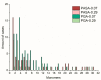
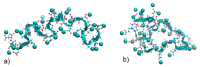


Similar articles
-
Effects of Amino Acid Side-Chain Length and Chemical Structure on Anionic Polyglutamic and Polyaspartic Acid Cellulose-Based Polyelectrolyte Brushes.Polymers (Basel). 2021 May 28;13(11):1789. doi: 10.3390/polym13111789. Polymers (Basel). 2021. PMID: 34071693 Free PMC article.
-
Changes in the Local Conformational States Caused by Simple Na+ and K+ Ions in Polyelectrolyte Simulations: Comparison of Seven Force Fields with and without NBFIX and ECC Corrections.Polymers (Basel). 2022 Jan 8;14(2):252. doi: 10.3390/polym14020252. Polymers (Basel). 2022. PMID: 35054659 Free PMC article.
-
Effect of side-chain length on the side-chain dynamics of alpha-helical poly(L-glutamic acid) as probed by a fluorescence blob model.J Phys Chem B. 2008 Jul 31;112(30):9209-18. doi: 10.1021/jp8021248. Epub 2008 Jul 9. J Phys Chem B. 2008. PMID: 18610962
-
Atomistic simulations of dilute polyelectrolyte solutions.J Phys Chem B. 2012 Apr 12;116(14):4319-27. doi: 10.1021/jp208138t. Epub 2012 Apr 3. J Phys Chem B. 2012. PMID: 22432449
-
To switch or not to switch: the effects of potassium and sodium ions on alpha-poly-L-glutamate conformations in aqueous solutions.J Am Chem Soc. 2009 Aug 12;131(31):10854-6. doi: 10.1021/ja9030374. J Am Chem Soc. 2009. PMID: 19618952
Cited by
-
Effects of Amino Acid Side-Chain Length and Chemical Structure on Anionic Polyglutamic and Polyaspartic Acid Cellulose-Based Polyelectrolyte Brushes.Polymers (Basel). 2021 May 28;13(11):1789. doi: 10.3390/polym13111789. Polymers (Basel). 2021. PMID: 34071693 Free PMC article.
References
-
- Wilts E.M., Herzberger J., Long T.E. Addressing water scarcity: Cationic polyelectrolytes in water treatment and purification. Polym. Int. 2018;67:799–814. doi: 10.1002/pi.5569. - DOI
-
- Migahed M.A., Rashwan S.M., Kamel M.M., Habib R.E. Synthesized polyaspartic acid derivatives as corrosion and scale inhibitors in desalination operations. Cogent Eng. 2017;4:1366255. doi: 10.1080/23311916.2017.1366255. - DOI
-
- De Geest B.G., De Koker S., Sukhorukov G.B., Kreft O., Parak W.J., Skirtach A.G., Demeester J., De Smedt S.C., Hennink W.E. Polyelectrolyte microcapsules for biomedical applications. Soft Matter. 2009;5:282–291. doi: 10.1039/B808262F. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

