Vaccine Advances against Venezuelan, Eastern, and Western Equine Encephalitis Viruses
- PMID: 32503232
- PMCID: PMC7350001
- DOI: 10.3390/vaccines8020273
Vaccine Advances against Venezuelan, Eastern, and Western Equine Encephalitis Viruses
Abstract
Vaccinations are a crucial intervention in combating infectious diseases. The three neurotropic Alphaviruses, Eastern (EEEV), Venezuelan (VEEV), and Western (WEEV) equine encephalitis viruses, are pathogens of interest for animal health, public health, and biological defense. In both equines and humans, these viruses can cause febrile illness that may progress to encephalitis. Currently, there are no licensed treatments or vaccines available for these viruses in humans. Experimental vaccines have shown variable efficacy and may cause severe adverse effects. Here, we outline recent strategies used to generate vaccines against EEEV, VEEV, and WEEV with an emphasis on virus-vectored and plasmid DNA delivery. Despite candidate vaccines protecting against one of the three viruses, few studies have demonstrated an effective trivalent vaccine. We evaluated the potential of published vaccines to generate cross-reactive protective responses by comparing DNA vaccine sequences to a set of EEEV, VEEV, and WEEV genomes and determining the vaccine coverages of potential epitopes. Finally, we discuss future directions in the development of vaccines to combat EEEV, VEEV, and WEEV.
Keywords: Alphavirus; DNA vaccine; Eastern equine encephalitis virus (EEEV); Venezuelan equine encephalitis virus (VEEV); Western equine encephalitis virus (WEEV); antigens; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. LA-UR-20-20011. This report was prepared as an account of work sponsored by an agency of the US Government. Neither Triad National Security, LLC, the US Government, nor any agency thereof, nor any of their employees make any warranty, express or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed or represent that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by Triad National Security, LLC, the US Government, or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of Triad National Security, LLC, the US Government, or any agency thereof.
Figures

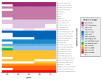

References
-
- Zumla A., Dar O., Kock R., Muturi M., Ntoumi F., Kaleebu P., Eusebio M., Mfinanga S., Bates M., Mwaba P., et al. Taking forward a ‘One Health’ approach for turning the tide against the Middle East respiratory syndrome coronavirus and other zoonotic pathogens with epidemic potential. Int. J. Infect. Dis. 2016;47:5–9. doi: 10.1016/j.ijid.2016.06.012. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

