In Vitro Effects of Ligand Bias on Primate Mu Opioid Receptor Downstream Signaling
- PMID: 32503269
- PMCID: PMC7312292
- DOI: 10.3390/ijms21113999
In Vitro Effects of Ligand Bias on Primate Mu Opioid Receptor Downstream Signaling
Abstract
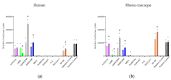
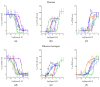
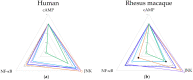
Interest has emerged in biased agonists at the mu opioid receptor (MOR) as a possible means for maintaining potent analgesis with reduced side effect profiles. While approaches measuring in vitro biased agonism are used in the development of these compounds, their therapeutic utility will ultimately be determined by in vivo functional effects. Nonhuman primates (NHPs) are the most translational model for evaluating the behavioral effects of candidate medications, but biased signaling of these drugs at NHP MOR receptors has been unstudied. The goal of the current work was to characterize MOR ligand bias in rhesus macaques, focusing on agonists that have previously been reported to show different patterns of biased agonism in rodents and humans. Downstream signaling pathways that responded to MOR activation were identified using a luciferase reporter array. Concentration-response curves for specific pathways (cAMP, NF-ĸB, MAPK/JNK) were generated using six agonists previously reported to differ in terms of signaling bias at rodent and human MORs. Using DAMGO as a reference ligand, relative cAMP, NF-ĸB and MAPK/JNK signaling by morphine, endomorphin-1, and TRV130 were found to be comparable between species. Further, the bias patterns of across ligands for NF-ĸB and MAPK/JNK were largely similar between species. There was a high degree of concordance between rhesus macaque and human MOR receptor signaling bias for all agonists tested, further demonstrating their utility for future translational behavioral studies.
Keywords: biased agonism; morphine; nonhuman primate; opioid; second messenger signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

