Sildenafil Recovers Burn-Induced Cardiomyopathy
- PMID: 32503314
- PMCID: PMC7349507
- DOI: 10.3390/cells9061393
Sildenafil Recovers Burn-Induced Cardiomyopathy
Abstract
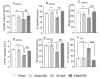
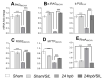
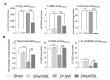
Background: Severe burn injury initiates a feedback cycle of inflammation, fibrosis, oxidative stress and cardiac mitochondrial damage via the PDE5A-cGMP-PKG pathway. Aim: To test if the PDE5A-cGMP-PKG pathway may contribute to burn-induced heart dysfunction. Methods: Sprague-Dawley rats were divided four groups: sham; sham/sildenafil; 24 h post burn (60% total body surface area scald burn, harvested at 24 h post burn); and 24 h post burn/sildenafil. We monitored heart function and oxidative adducts, as well as cardiac inflammatory, cardiac fibrosis and cardiac remodeling responses in vivo. Results: Sildenafil inhibited the burn-induced PDE5A mRNA level and increased the cGMP level and PKG activity, leading to the normalization of PKG down-regulated genes (IRAG, PLB, RGS2, RhoA and MYTP), a decreased ROS level (H2O2), decreased oxidatively modified adducts (malonyldialdehyde [MDA], carbonyls), attenuated fibrogenesis as well as fibrosis gene expression (ANP, BNP, COL1A2, COL3A2, αSMA and αsk-Actin), and reduced inflammation and related gene expression (RELA, IL-18 and TGF-β) after the burn. Additionally, sildenafil treatment preserved left ventricular heart function (CO, EF, SV, LVvol at systolic, LVPW at diastolic and FS) and recovered the oxidant/antioxidant balance (total antioxidant, total SOD activity and Cu,ZnSOD activity). Conclusions: The PDE5A-cGMP-PKG pathway mediates burn-induced heart dysfunction. Sildenafil treatment recovers burn-induced cardiac dysfunction.
Keywords: PDE5A; burn injury; cardiomyopathy; fibrogenesis; oxidative stress; sildenafil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Burn-Induced Cardiac Mitochondrial Dysfunction via Interruption of the PDE5A-cGMP-PKG Pathway.Int J Mol Sci. 2020 Mar 28;21(7):2350. doi: 10.3390/ijms21072350. Int J Mol Sci. 2020. PMID: 32231130 Free PMC article.
-
PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation.Basic Res Cardiol. 2010 May;105(3):337-47. doi: 10.1007/s00395-010-0084-5. Epub 2010 Jan 27. Basic Res Cardiol. 2010. PMID: 20107996 Free PMC article.
-
Distinct phosphodiesterase 5A-containing compartments allow selective regulation of cGMP-dependent signalling in human arterial smooth muscle cells.Cell Signal. 2017 Aug;36:204-211. doi: 10.1016/j.cellsig.2017.04.019. Epub 2017 May 12. Cell Signal. 2017. PMID: 28506928
-
Cyclic GMP-dependent signaling in cardiac myocytes.Circ J. 2012;76(8):1819-25. doi: 10.1253/circj.cj-12-0664. Epub 2012 Jul 29. Circ J. 2012. PMID: 22785374 Review.
-
The NO-cGMP-PKG pathway in skeletal remodeling.Ann N Y Acad Sci. 2021 Mar;1487(1):21-30. doi: 10.1111/nyas.14486. Epub 2020 Aug 28. Ann N Y Acad Sci. 2021. PMID: 32860248 Free PMC article. Review.
Cited by
-
Myocardial Impact of NHE1 Regulation by Sildenafil.Front Cardiovasc Med. 2021 Feb 22;8:617519. doi: 10.3389/fcvm.2021.617519. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33693035 Free PMC article. Review.
-
Study on metabolic disorders in rat liver induced by different times after scalds.Biochem Biophys Rep. 2024 Dec 21;41:101904. doi: 10.1016/j.bbrep.2024.101904. eCollection 2025 Mar. Biochem Biophys Rep. 2024. PMID: 39811192 Free PMC article.
-
Novel Functional Features of cGMP Substrate Proteins IRAG1 and IRAG2.Int J Mol Sci. 2023 Jun 7;24(12):9837. doi: 10.3390/ijms24129837. Int J Mol Sci. 2023. PMID: 37372987 Free PMC article. Review.
-
Effect of Mitochondrial Antioxidant (Mito-TEMPO) on Burn-Induced Cardiac Dysfunction.J Am Coll Surg. 2021 Apr;232(4):642-655. doi: 10.1016/j.jamcollsurg.2020.11.031. Epub 2021 Jan 7. J Am Coll Surg. 2021. PMID: 33421567 Free PMC article.
-
Exploring the application of sildenafil for high-fat diet-induced erectile dysfunction based on interleukin-18-mediated NLRP3/Caspase-1 signaling pathway.Sex Med. 2023 Aug 25;11(4):qfad044. doi: 10.1093/sexmed/qfad044. eCollection 2023 Aug. Sex Med. 2023. PMID: 37636019 Free PMC article.
References
-
- WHO Burns. [(accessed on 2 June 2020)]; Available online: https://www.who.int/violence_injury_prevention/other_injury/burns/en/
-
- CDC Burn Prevention. [(accessed on 2 June 2020)]; Available online: https://www.cdc.gov/safechild/burns/index.html.
-
- Lawrence B.A., Zaloshnja E., Miller T.R., Jones P.R. Estimates of the Incidence and Costs of Fire-Related Injuries. The U.S. Consumer Product Safety Commission; Calveston, MD, USA: 2009. pp. 1–51.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

