Microtubule Organization in Striated Muscle Cells
- PMID: 32503326
- PMCID: PMC7349303
- DOI: 10.3390/cells9061395
Microtubule Organization in Striated Muscle Cells
Abstract
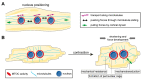
Distinctly organized microtubule networks contribute to the function of differentiated cell types such as neurons, epithelial cells, skeletal myotubes, and cardiomyocytes. In striated (i.e. skeletal and cardiac) muscle cells, the nuclear envelope acts as the dominant microtubule-organizing center (MTOC) and the function of the centrosome-the canonical MTOC of mammalian cells-is attenuated, a common feature of differentiated cell types. We summarize the mechanisms known to underlie MTOC formation at the nuclear envelope, discuss the significance of the nuclear envelope MTOC for muscle function and cell cycle progression, and outline potential mechanisms of centrosome attenuation.
Keywords: MTOC; cardiomyocytes; cell cycle; centrosome; microtubules; non-centrosomal MTOC; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zebrowski D.C., Vergarajauregui S., Wu C.C., Piatkowski T., Becker R., Leone M., Hirth S., Ricciardi F., Falk N., Giessl A., et al. Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. Elife. 2015;4 doi: 10.7554/eLife.05563. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

