Short-term efficacy and safety of lasmiditan, a novel 5-HT1F receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis
- PMID: 32503415
- PMCID: PMC7275414
- DOI: 10.1186/s10194-020-01138-x
Short-term efficacy and safety of lasmiditan, a novel 5-HT1F receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis
Abstract
Background: Migraine has been recognized as one of common diseases in the world whose current treatment options are not ideal. Lasmiditan, an oral 5-hydroxytryptamine (HT)1F receptor agonist, appears more promising for the acute treatment of migraine because of considerably better effect profiles with no severe adverse events (AEs). This review aimed to systematically evaluate the efficacy and safety of lasmiditan from the results of randomized controlled trials (RCTs).
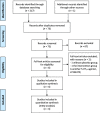
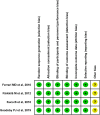
Methods: PubMed, Cochrane Library, Embase were searched on lasmiditan for the acute treatment of migraine from inception of the databases to Feb 1, 2020. Pain free and pain relief, global impression (very much/much better), and no/mild disability at 2 h in efficacy; total treatment-emergent adverse events (TEAEs), dizziness, nausea, fatigue, paraesthesia and somnolence in safety were extracted from the included studies. A systematic review and meta-analysis was performed using Review Manager Software version 5.3 (RevMan 5.3).
Results: Four RCTs with a total of 4960 subjects met our inclusion criteria. The overall effect estimate showed that lasmiditan was significantly superior to placebo in terms of pain free (RR 1.71, 95% CI 1.55-1.87), pain relief (RR 1.40, 95% CI 1.33-1.47), global impression (very much/much better) (RR 1.55, 95% CI 1.44-1.67), and no/mild disability (RR 1.15, 95% CI 1.10-1.20) at 2 h. For the safety, significant number of patients experienced TEAEs with lasmiditan than with placebo (RR 2.77, 95% CI 2.53-3.03), most TEAEs were central nervous system (CNS)-related and included dizziness (RR 5.81, 95% CI 4.72-7.14), nausea (RR 2.58, 95% CI 1.87-3.57), fatigue (RR 5.38, 95% CI 3.78-7.66), paraesthesia (RR 4.48, 95% CI 3.33-6.02), and somnolence (RR 2.82, 95% CI 2.18-3.66).
Conclusions: This meta-analysis suggests that lasmiditan is effective for the acute treatment of migraine with a higher incidence of CNS-related adverse reactions compared with placebo. Long-term, open-label, multi-dose trials are required to verify the current findings.
Keywords: 5-HT1F receptor agonist; Lasmiditan; Meta-analysis; Migraine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- GBD Disease and injury incidence and prevalence collaborators (2017) global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2016;390(10100):1211–1259. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

