Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis
- PMID: 32503598
- PMCID: PMC7275323
- DOI: 10.1186/s13023-020-01430-8
Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis
Abstract
Background: Duchenne Muscular Dystrophy (DMD) is a rare disorder caused by mutations in the dystrophin gene. A recent systematic review and meta-analysis of global DMD epidemiology is not available. This study aimed to estimate the global overall and birth prevalence of DMD through an updated systematic review of the literature.
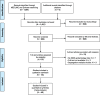
Methods: MEDLINE and EMBASE databases were searched for original research articles on the epidemiology of DMD from inception until 1st October 2019. Studies were included if they were original observational research articles written in English, reporting DMD prevalence and/or incidence along with the number of individuals of the underlying population. The quality of the studies was assessed using a STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist adapted for observational studies on rare diseases. To derive the pooled epidemiological prevalence estimates, a meta-analysis was performed using random-effects logistic models for overall and birth prevalence and within two different underlying populations (i.e. all individuals and in males only), separately. Heterogeneity was assessed using Cochran's Q-test along with its derived measure of inconsistency I2.
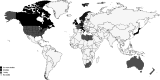
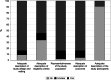
Results: A total of 44 studies reporting the global epidemiology of DMD were included in the systematic review and only 40 were included in the meta-analysis. The pooled global DMD prevalence was 7.1 cases (95% CI: 5.0-10.1) per 100,000 males and 2.8 cases (95% CI: 1.6-4.6) per 100,000 in the general population, while the pooled global DMD birth prevalence was 19.8 (95% CI:16.6-23.6) per 100,000 live male births. A very high between-study heterogeneity was found for each epidemiological outcome and for all underlying populations (I2 > 90%). The test for funnel plot asymmetry suggested the absence of publication bias. Of the 44 studies included in this systematic review, 36 (81.8%) were assessed as being of medium and 8 (18.2%) of low quality, while no study was assessed as being of high quality.
Conclusions: Generating epidemiological evidence on DMD is fundamental to support public health decision-making. The high heterogeneity and the lack of high quality studies highlights the need to conduct better quality studies on rare diseases.
Keywords: Birth prevalence; Duchenne muscular dystrophy; Epidemiology; Meta-analysis; Prevalence; Systematic review.
Conflict of interest statement
G. Trifirò has served on advisory boards for Sandoz, Hospira, Sanofi, Biogen, Ipsen, and Shire and is a consultant for Otsuka. G Trifirò is the principal investigator of observational studies funded by several pharmaceutical companies (e.g. Amgen, AstraZeneca, Daiichi Sankyo and IBSA) to University of Messina, as well as scientific coordinator of the Master’s program ‘Pharmacovigilance, pharmacoepidemiology and pharmacoeconomics: real-world data evaluations’ at University of Messina, which is partly funded by several pharmaceutical companies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures



References
-
- Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Glob Health. 2018;4422(18). 10.1016/S1474-4422(18)30024-3. - PMC - PubMed
-
- Giliberto F, Radic CP, Luce L, Ferreiro V, de Brasi C, Szijan I. Symptomatic female carriers of Duchenne muscular dystrophy (DMD): genetic and clinical characterization. J Neurol Sci. 2014;336(1–2):36–41. - PubMed
-
- Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–929. - PubMed
-
- Eagle M, Bourke J, Bullock R, Gibson M, Mehta J, Giddings D, et al. Managing Duchenne muscular dystrophy - the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord. 2007;17(6):470–475. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

