Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders
- PMID: 32503641
- PMCID: PMC7275301
- DOI: 10.1186/s13024-020-00375-7
Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders
Abstract
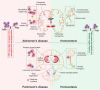
Emerging evidence demonstrates that adaptive immunity influences the pathobiology of neurodegenerative disorders. Misfolded aggregated self-proteins can break immune tolerance leading to the induction of autoreactive effector T cells (Teffs) with associated decreases in anti-inflammatory neuroprotective regulatory T cells (Tregs). An imbalance between Teffs and Tregs leads to microglial activation, inflammation and neuronal injury. The cascade of such a disordered immunity includes the drainage of the aggregated protein antigens into cervical lymph nodes serving to amplify effector immune responses. Both preclinical and clinical studies demonstrate transformation of this altered immunity for therapeutic gain. We posit that the signs and symptoms of common neurodegenerative disorders such as Alzheimer's and Parkinson's diseases, amyotrophic lateral sclerosis, and stroke can be attenuated by boosting Treg activities.
Keywords: Dendritic cells; Effector T cells (Teffs); Immune transformation; Microglia; Neurodegenerative disorders; Regulatory T cells (Tregs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

