Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine
- PMID: 32503843
- PMCID: PMC7383395
- DOI: 10.1074/jbc.REV120.011188
Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine
Abstract
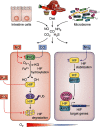
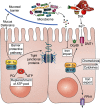
The gastrointestinal tract is a highly proliferative and regenerative tissue. The intestine also harbors a large and diverse microbial population collectively called the gut microbiome (microbiota). The microbiome-intestine cross-talk includes a dynamic exchange of gaseous signaling mediators generated by bacterial and intestinal metabolisms. Moreover, the microbiome initiates and maintains the hypoxic environment of the intestine that is critical for nutrient absorption, intestinal barrier function, and innate and adaptive immune responses in the mucosal cells of the intestine. The response to hypoxia is mediated by hypoxia-inducible factors (HIFs). In hypoxic conditions, the HIF activation regulates the expression of a cohort of genes that promote adaptation to hypoxia. Physiologically, HIF-dependent genes contribute to the aforementioned maintenance of epithelial barrier function, nutrient absorption, and immune regulation. However, chronic HIF activation exacerbates disease conditions, leading to intestinal injury, inflammation, and colorectal cancer. In this review, we aim to outline the major roles of physiological and pathological hypoxic conditions in the maintenance of intestinal homeostasis and in the onset and progression of disease with a major focus on understanding the complex pathophysiology of the intestine.
Keywords: HIF-2α; colitis; colon cancer; hypoxia; hypoxia-inducible factor (HIF); hypoxia-inducible factor 1α (HIF-1α); inflammation; inflammatory bowel disease (IBD); intestinal epithelium; intestinal metabolism; iron; iron metabolism; mucosal barrier.
© 2020 Singhal and Shah.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- He G., Shankar R. A., Chzhan M., Samouilov A., Kuppusamy P., and Zweier J. L. (1999) Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. U. S. A. 96, 4586–4591 10.1073/pnas.96.8.4586 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

