T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection
- PMID: 32503876
- PMCID: PMC7416530
- DOI: 10.1126/sciimmunol.abb1817
T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection
Abstract
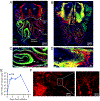
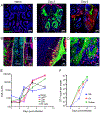
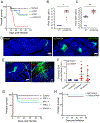
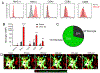
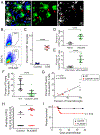
The neuroepithelium is a nasal barrier surface populated by olfactory sensory neurons that detect odorants in the airway and convey this information directly to the brain via axon fibers. This barrier surface is especially vulnerable to infection, yet respiratory infections rarely cause fatal encephalitis, suggesting a highly evolved immunological defense. Here, using a mouse model, we sought to understand the mechanism by which innate and adaptive immune cells thwart neuroinvasion by vesicular stomatitis virus (VSV), a potentially lethal virus that uses olfactory sensory neurons to enter the brain after nasal infection. Fate-mapping studies demonstrated that infected central nervous system (CNS) neurons were cleared noncytolytically, yet specific deletion of major histocompatibility complex class I (MHC I) from these neurons unexpectedly had no effect on viral control. Intravital imaging studies of calcium signaling in virus-specific CD8+ T cells revealed instead that brain-resident microglia were the relevant source of viral peptide-MHC I complexes. Microglia were not infected by the virus but were found to cross-present antigen after acquisition from adjacent neurons. Microglia depletion interfered with T cell calcium signaling and antiviral control in the brain after nasal infection. Collectively, these data demonstrate that microglia provide a front-line defense against a neuroinvasive nasal infection by cross-presenting antigen to antiviral T cells that noncytolytically cleanse neurons. Disruptions in this innate defense likely render the brain susceptible to neurotropic viruses like VSV that attempt to enter the CNS via the nose.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials