Cell-Based Phenotypic Drug Screening Identifies Luteolin as Candidate Therapeutic for Nephropathic Cystinosis
- PMID: 32503896
- PMCID: PMC7351012
- DOI: 10.1681/ASN.2019090956
Cell-Based Phenotypic Drug Screening Identifies Luteolin as Candidate Therapeutic for Nephropathic Cystinosis
Abstract
Background: Mutations in the gene that encodes the lysosomal cystine transporter cystinosin cause the lysosomal storage disease cystinosis. Defective cystine transport leads to intralysosomal accumulation and crystallization of cystine. The most severe phenotype, nephropathic cystinosis, manifests during the first months of life, as renal Fanconi syndrome. The cystine-depleting agent cysteamine significantly delays symptoms, but it cannot prevent progression to ESKD and does not treat Fanconi syndrome. This suggests the involvement of pathways in nephropathic cystinosis that are unrelated to lysosomal cystine accumulation. Recent data indicate that one such potential pathway, lysosome-mediated degradation of autophagy cargoes, is compromised in cystinosis.
Methods: To identify drugs that reduce levels of the autophagy-related protein p62/SQSTM1 in cystinotic proximal tubular epithelial cells, we performed a high-throughput screening on the basis of an in-cell ELISA assay. We then tested a promising candidate in cells derived from patients with, and mouse models of, cystinosis, and in preclinical studies in cystinotic zebrafish.
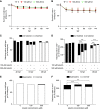
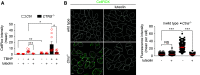
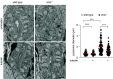
Results: Of 46 compounds identified as reducing p62/SQSTM1 levels in cystinotic cells, we selected luteolin on the basis of its efficacy, safety profile, and similarity to genistein, which we previously showed to ameliorate other lysosomal abnormalities of cystinotic cells. Our data show that luteolin improves the autophagy-lysosome degradative pathway, is a powerful antioxidant, and has antiapoptotic properties. Moreover, luteolin stimulates endocytosis and improves the expression of the endocytic receptor megalin.
Conclusions: Our data show that luteolin improves defective pathways of cystinosis and has a good safety profile, and thus has potential as a treatment for nephropathic cystinosis and other renal lysosomal storage diseases.
Keywords: apoptosis; autophagy; endocytosis; lysosomal storage disease; reactive.
Copyright © 2020 by the American Society of Nephrology.
Figures






Similar articles
-
Long-term effects of luteolin in a mouse model of nephropathic cystinosis.Biomed Pharmacother. 2024 Sep;178:117236. doi: 10.1016/j.biopha.2024.117236. Epub 2024 Aug 2. Biomed Pharmacother. 2024. PMID: 39096619
-
Protection of Cystinotic Mice by Kidney-Specific Megalin Ablation Supports an Endocytosis-Based Mechanism for Nephropathic Cystinosis Progression.J Am Soc Nephrol. 2019 Nov;30(11):2177-2190. doi: 10.1681/ASN.2019040371. Epub 2019 Sep 23. J Am Soc Nephrol. 2019. PMID: 31548351 Free PMC article.
-
Use of Human Induced Pluripotent Stem Cells and Kidney Organoids To Develop a Cysteamine/mTOR Inhibition Combination Therapy for Cystinosis.J Am Soc Nephrol. 2020 May;31(5):962-982. doi: 10.1681/ASN.2019070712. Epub 2020 Mar 20. J Am Soc Nephrol. 2020. PMID: 32198276 Free PMC article.
-
Effects of long-term cysteamine treatment in patients with cystinosis.Pediatr Nephrol. 2019 Apr;34(4):571-578. doi: 10.1007/s00467-017-3856-4. Epub 2017 Dec 19. Pediatr Nephrol. 2019. PMID: 29260317 Free PMC article. Review.
-
Nephropathic cystinosis: an update on genetic conditioning.Pediatr Nephrol. 2021 Jun;36(6):1347-1352. doi: 10.1007/s00467-020-04638-9. Epub 2020 Jun 20. Pediatr Nephrol. 2021. PMID: 32564281 Review.
Cited by
-
Drug Repurposing in Rare Diseases: An Integrative Study of Drug Screening and Transcriptomic Analysis in Nephropathic Cystinosis.Int J Mol Sci. 2021 Nov 27;22(23):12829. doi: 10.3390/ijms222312829. Int J Mol Sci. 2021. PMID: 34884638 Free PMC article.
-
Luteolin and its antidepressant properties: From mechanism of action to potential therapeutic application.J Pharm Anal. 2025 Apr;15(4):101097. doi: 10.1016/j.jpha.2024.101097. Epub 2024 Sep 7. J Pharm Anal. 2025. PMID: 40276566 Free PMC article. Review.
-
Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives.Cancers (Basel). 2022 Oct 31;14(21):5373. doi: 10.3390/cancers14215373. Cancers (Basel). 2022. PMID: 36358791 Free PMC article. Review.
-
Genistein improves renal disease in a mouse model of nephropathic cystinosis: a comparison study with cysteamine.Hum Mol Genet. 2023 Mar 20;32(7):1090-1101. doi: 10.1093/hmg/ddac266. Hum Mol Genet. 2023. PMID: 36300303 Free PMC article.
-
Multisystem involvement, defective lysosomes and impaired autophagy in a novel rat model of nephropathic cystinosis.Hum Mol Genet. 2022 Jul 7;31(13):2262-2278. doi: 10.1093/hmg/ddac033. Hum Mol Genet. 2022. PMID: 35137071 Free PMC article.
References
-
- Gahl WA, Thoene JG, Schneider JA: Cystinosis. N Engl J Med 347: 111–121, 2002. - PubMed
-
- Brodin-Sartorius A, Tête MJ, Niaudet P, Antignac C, Guest G, Ottolenghi C, et al. .: Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int 81: 179–189, 2012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

