Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity
- PMID: 32503945
- PMCID: PMC7279670
- DOI: 10.1136/jitc-2019-000266
Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity
Abstract
Background: The use of checkpoint inhibitors has revolutionized cancer therapy. Unfortunately, these therapies often cause immune-related adverse effects, largely due to a lack of tumor specificity.
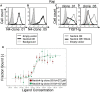
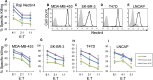
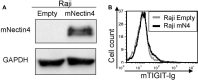
Methods: We stained human natural killer cells using fusion proteins composed of the extracellular portion of various tumor markers fused to the Fc portion of human IgG1, and identified Nectin4 as a novel TIGIT ligand. Next, we generated a novel Nectin4 blocking antibody and demonstrated its efficacy as a checkpoint inhibitor in killing assays and in vivo.
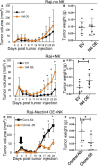
Results: We identify Nectin4 to be a novel ligand of TIGIT. We showed that, as opposed to all other known TIGIT ligands, which bind also additional receptors, Nectin4 interacts only with TIGIT. We show that the TIGIT-Nectin4 interaction inhibits natural killer cell activity, a critical part of the innate immune response. Finally, we developed blocking Nectin4 antibodies and demonstrated that they enhance tumor killing in vitro and in vivo.
Conclusion: We discovered that Nectin4 is a novel ligand for TIGIT and demonstrated that specific antibodies against it enhance tumor cell killing in vitro and in vivo. Since Nectin4 is expressed almost exclusively on tumor cells, our Nectin4-blocking antibodies represent a combination of cancer specificity and immune checkpoint activity, which may prove more effective and safe for cancer immunotherapy.
Keywords: immunology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
