The effect of flow on swimming bacteria controls the initial colonization of curved surfaces
- PMID: 32503979
- PMCID: PMC7275075
- DOI: 10.1038/s41467-020-16620-y
The effect of flow on swimming bacteria controls the initial colonization of curved surfaces
Abstract
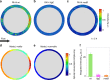
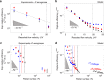
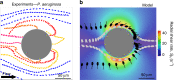
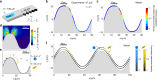
The colonization of surfaces by bacteria is a widespread phenomenon with consequences on environmental processes and human health. While much is known about the molecular mechanisms of surface colonization, the influence of the physical environment remains poorly understood. Here we show that the colonization of non-planar surfaces by motile bacteria is largely controlled by flow. Using microfluidic experiments with Pseudomonas aeruginosa and Escherichia coli, we demonstrate that the velocity gradients created by a curved surface drive preferential attachment to specific regions of the collecting surface, namely the leeward side of cylinders and immediately downstream of apexes on corrugated surfaces, in stark contrast to where nonmotile cells attach. Attachment location and rate depend on the local hydrodynamics and, as revealed by a mathematical model benchmarked on the observations, on cell morphology and swimming traits. These results highlight the importance of flow on the magnitude and location of bacterial colonization of surfaces.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Flemming H-C, et al. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. - PubMed
-
- Hall-stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. - PubMed
-
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. - PubMed
-
- Flemming HC. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002;59:629–640. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

