Multidimensional imaging of liver injury repair in mice reveals fundamental role of the ductular reaction
- PMID: 32503996
- PMCID: PMC7275065
- DOI: 10.1038/s42003-020-1006-1
Multidimensional imaging of liver injury repair in mice reveals fundamental role of the ductular reaction
Abstract
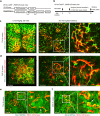
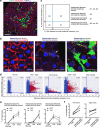
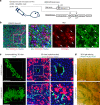
Upon severe and/or chronic liver injury, ectopic emergence and expansion of atypical biliary epithelial-like cells in the liver parenchyma, known as the ductular reaction, is typically induced and implicated in organ regeneration. Although this phenomenon has long been postulated to represent activation of facultative liver stem/progenitor cells that give rise to new hepatocytes, recent lineage-tracing analyses have challenged this notion, thereby leaving the pro-regenerative role of the ductular reaction enigmatic. Here, we show that the expanded and remodelled intrahepatic biliary epithelia in the ductular reaction constituted functional and complementary bile-excreting conduit systems in injured parenchyma where hepatocyte bile canalicular networks were lost. The canalicular collapse was an incipient defect commonly associated with hepatocyte injury irrespective of cholestatic statuses, and could sufficiently provoke the ductular reaction when artificially induced. We propose a unifying model for the induction of the ductular reaction, where compensatory biliary epithelial tissue remodeling ensures bile-excreting network homeostasis.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The transcription factor Klf5 is essential for intrahepatic biliary epithelial tissue remodeling after cholestatic liver injury.J Biol Chem. 2018 Apr 27;293(17):6214-6229. doi: 10.1074/jbc.RA118.002372. Epub 2018 Mar 9. J Biol Chem. 2018. PMID: 29523685 Free PMC article.
-
Ductular hepatocytes.Histol Histopathol. 1995 Apr;10(2):433-56. Histol Histopathol. 1995. PMID: 7599440 Review.
-
Loss of keratin 19 favours the development of cholestatic liver disease through decreased ductular reaction.J Pathol. 2015 Nov;237(3):343-54. doi: 10.1002/path.4580. Epub 2015 Aug 3. J Pathol. 2015. PMID: 26108453
-
Contributions of hepatocytes and bile ductular cells in ductular reactions and remodeling of the biliary system after chronic liver injury.Am J Pathol. 2014 Nov;184(11):3001-12. doi: 10.1016/j.ajpath.2014.07.005. Epub 2014 Sep 2. Am J Pathol. 2014. PMID: 25193593
-
Development and molecular composition of the hepatic progenitor cell niche.Dan Med J. 2013 May;60(5):B4640. Dan Med J. 2013. PMID: 23673270 Review.
Cited by
-
Panic at the Bile Duct: How Intrahepatic Cholangiocytes Respond to Stress and Injury.Am J Pathol. 2023 Oct;193(10):1440-1454. doi: 10.1016/j.ajpath.2023.02.012. Epub 2023 Mar 2. Am J Pathol. 2023. PMID: 36870530 Free PMC article. Review.
-
β-Catenin-NF-κB-CFTR interactions in cholangiocytes regulate inflammation and fibrosis during ductular reaction.Elife. 2021 Oct 5;10:e71310. doi: 10.7554/eLife.71310. Elife. 2021. PMID: 34609282 Free PMC article.
-
Development and evaluation of Hsp90-targeting nanobodies for visualisation of extracellular Hsp90 in tumours using PET imaging.EJNMMI Radiopharm Chem. 2025 Feb 21;10(1):10. doi: 10.1186/s41181-025-00331-0. EJNMMI Radiopharm Chem. 2025. PMID: 39982615 Free PMC article.
-
Altered Hepatic Metabolism in Down Syndrome.bioRxiv [Preprint]. 2025 May 31:2025.05.27.656393. doi: 10.1101/2025.05.27.656393. bioRxiv. 2025. PMID: 40502193 Free PMC article. Preprint.
-
BiliQML: a supervised machine-learning model to quantify biliary forms from digitized whole slide liver histopathological images.Am J Physiol Gastrointest Liver Physiol. 2024 Jul 1;327(1):G1-G15. doi: 10.1152/ajpgi.00058.2024. Epub 2024 Apr 23. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38651949 Free PMC article.
References
-
- Kopp JL, Grompe M, Sander M. Stem cells versus plasticity in liver and pancreas regeneration. Nat. Cell Biol. 2016;18:238–245. - PubMed
-
- Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol. Res. Pr. 1995;191:513–524. - PubMed
-
- Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nat. Rev. Gastroenterol. Hepatol. 2016;13:473–485. - PubMed
-
- Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

