Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group
- PMID: 32504038
- PMCID: PMC7337227
- DOI: 10.1038/s41433-020-0961-6
Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group
Erratum in
-
Correction: Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group.Eye (Lond). 2020 Oct;34(10):1941-1942. doi: 10.1038/s41433-020-1087-6. Eye (Lond). 2020. PMID: 32699378 Free PMC article.
Abstract
The management of diabetic retinopathy (DR) has evolved considerably over the past decade, with the availability of new technologies (diagnostic and therapeutic). As such, the existing Royal College of Ophthalmologists DR Guidelines (2013) are outdated, and to the best of our knowledge are not under revision at present. Furthermore, there are no other UK guidelines covering all available treatments, and there seems to be significant variation around the UK in the management of diabetic macular oedema (DMO). This manuscript provides a summary of reviews the pathogenesis of DR and DMO, including role of vascular endothelial growth factor (VEGF) and non-VEGF cytokines, clinical grading/classification of DMO vis a vis current terminology (of centre-involving [CI-DMO], or non-centre involving [nCI-DMO], systemic risks and their management). The excellent UK DR Screening (DRS) service has continued to evolve and remains world-leading. However, challenges remain, as there are significant variations in equipment used, and reproducible standards of DMO screening nationally. The interphase between DRS and the hospital eye service can only be strengthened with further improvements. The role of modern technology including optical coherence tomography (OCT) and wide-field imaging, and working practices including virtual clinics and their potential in increasing clinic capacity and improving patient experiences and outcomes are discussed. Similarly, potential roles of home monitoring in diabetic eyes in the future are explored. The role of pharmacological (intravitreal injections [IVT] of anti-VEGFs and steroids) and laser therapies are summarised. Generally, IVT anti-VEGF are offered as first line pharmacologic therapy. As requirements of diabetic patients in particular patient groups may vary, including pregnant women, children, and persons with learning difficulties, it is important that DR management is personalised in such particular patient groups. First choice therapy needs to be individualised in these cases and may be intravitreal steroids rather than the standard choice of anti-VEGF agents. Some of these, but not all, are discussed in this document.
Conflict of interest statement
WMA has received honoraria for advisory board memberships from AbbVie, Alcon, Alimera, Allergan, Bayer, Bausch and Lomb, Novartis and Pfizer, speaker Fees from Alimera, Allergan, Bayer, Novartis and Pfizer, and Educational travel grants from Alimera, Allergan, Bayer, Novartis and Pfizer. He has undertaken clinical research sponsored by Allergan, Bayer, Gyroscope, and Novartis. His institution has received research funding from Allergan, Bayer, Boehringer Ingelheim, CenterVue, Novartis, and Optos plc. CB attended advisory boards of, and received lecture fees from Novartis, Bayer, Roche, Allergan, Alimera Sciences. Her employer Bristol Eye Hospital has received research funding from Boehringer Ingelheim, Roche, Bayer, Novartis, and Allergan. Sanjiv Banerjee has received speaker fees and travel grants from Novartis, and honoraria for advisory board memberships and travel grants from Allergan. Somnath Banerjee has received honoraria for Advisory Boards from Bayer and Alimera, and Educational Travel Grants from Allergan, Alimera, and DORC. LD has received honoraria for advisory board memberships from Alimera, Allergan, Bayer, Novartis and Thrombogenics, speaker fees from Alimera, Bausch and Lomb, Bayer and Novartis and educational travel grants from Allergan, Bayer and Novartis. Her institution has undertaken clinical research sponsored by Alimera, Allergan, Bayer, Novartis and Roche. RG has received travel grants from Allergan, Bayer and Novartis and research grants from Novartis and Bayer. He has received honoraria for advisory board memberships from Bayer, Novartis, Allergan, Alimera, and Roche. FG has received honorarium for consultancy-advisory boards from Alimera, Allergan, Bayer, Novartis, Oxford BioElectronics, Roche; educational travel grants from Allergan, Bayer, Novartis and departmental research grants from Allergan, Bayer, Boehringer Ingelheim, Chengdu Pharma, Novartis, PanOptica. RH has received educational travel awards from Allergan, Bayer, and Novartis. He has served on advisory boards of Allergan, Bayer, Novartis, and Roche. His institution has received research funding from Allergan, Novartis, Roche, Bayer, Thea, and Thrombogenics. KK has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Berlin-Chemie AG/Menarini Group, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi, Takeda, Servier and Pfizer, research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Pfizer. HM has received research grants, educational travel grants and honoraria for advisory board memberships from Allergan, Bayer, Novartis and Roche. EP has received honoraria for advisory board memberships from Alimera, Allergan, Educational sponsorships from Alimera, Allergan, and Bayer, and speaker fees from Allergan, Novartis. Her institution has received research funding from Bayer. FQ has received honoraria for advisory board memberships and speaker fees from Alimera Sciences, Allergan, Heidelberg Engineering, SIFI, Meagate and Novartis. He has received educational travel grants from Alimera Sciences, Allergan, Heidelberg Engineering, SIFI, Meagate and Novartis. SR has no declarations. RS has received educational travel grants and speaker fees from Allergan, Bayer, and Novartis. DS has received speaker fees and travel grants from Allergan, Bayer, Novartis, and Roche, and honoraria for advisory board memberships from Allergan and Big Picture Medical UK. DV has received honoraria for advisory board meetings and speaker fees from Allergan, Bayer and Novartis; travel sponsorships from Alimera, Allergan, Bayer and Novartis. Her institution has undertaken clinical research sponsored by Allergan, Alimera, Bayer, Roche, Boehringer Ingelheim, Chengdu Kanghong Biotechnology Co Ltd and Novartis.
Figures


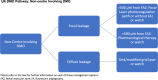

References
-
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. - PubMed
-
- Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic Macular Edema: Pathogenesis and Treatment. Survey of Ophthalmology. 2009;54:1–32. - PubMed
-
- Abbate M, Cravedi P, Iliev I, Remuzzi G, Ruggenenti P. Prevention and Treatment of Diabetic Retinopathy: Evidence from Clinical Trials and Perspectives. Current Diabetes Reviews. 2011;7:190–200. - PubMed
-
- Heng LZ, Comyn O, Peto T, Tadros C, Ng E, Sivaprasad S, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetic Medicine. 2013;30:640–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

