Distal Gastrectomy with Billroth II Reconstruction is Associated with Oralization of Gut Microbiome and Intestinal Inflammation: A Proof-of-Concept Study
- PMID: 32504369
- PMCID: PMC7801296
- DOI: 10.1245/s10434-020-08678-1
Distal Gastrectomy with Billroth II Reconstruction is Associated with Oralization of Gut Microbiome and Intestinal Inflammation: A Proof-of-Concept Study
Abstract
Background: Subtotal gastrectomy with Billroth II reconstruction (SGB2) results in increased gastric pH and diminished gastric barrier. Increased gastric pH following PPI therapy has an impact on the gut microbiome, intestinal inflammation, and possibly patient health. If similar changes are present after SGB2, these can be relevant for patient health and long-term outcomes after surgery. The aim of the study is to investigate whether SGB2 is associated with specific changes in gut microbiome composition and intestinal inflammation.
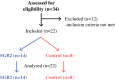
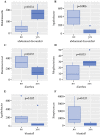
Patients and methods: This cross-sectional proof-of-concept study includes patients after SGB2 (n = 14) for early gastric cancer and their nongastrectomized in-house relatives as controls (n = 8). Fecal microbiome composition, intestinal inflammation (fecal calprotectin), gut permeability (DAO, LBP, sCD14), systemic inflammation (CRP) markers, and gastrointestinal symptoms are investigated. This study is registered at ClinicalTrials.gov (NCT03418428).
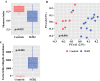
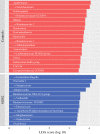
Results: Microbiome oralization following SGB2 was defined by an increase in Escherichia-Shigella, Enterococcus, Streptococcus, and other typical oral cavity bacteria (Veillonella, Oribacterium, and Mogibacterium) abundance. The fecal calprotectin was increased in the SGB2 group [100.9 (52.1; 292) vs. 25.8 (17; 66.5); p = 0.014], and calprotectin levels positively correlated with the abundance of Streptococcus (rs = 0.639; padj = 0.023). Gastrointestinal symptoms in SGB2 patients were associated with distinct taxonomic changes of the gut microbiome.
Conclusions: SGB2 is associated with oralization of the gut microbiome; intestinal inflammation and microbiome changes were associated with gastrointestinal symptoms. These novel findings may open gut microbiome as a new target for therapy to improve quality of life and general patient health in long-term survivors after SGB2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tran TB, Worhunsky DJ, Squires MH, Jin LX, Spolverato G, Votanopoulos KI, et al. To Roux or not to Roux: a comparison between Roux-en-Y and Billroth II reconstruction following partial gastrectomy for gastric cancer. Gastric Cancer. 2016;19(3):994–1001. doi: 10.1007/s10120-015-0547-3. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

