The Current Status of European and National Financial Sources for Clinical Research and Their Impact on Paediatric Non-commercial Clinical Trials: A Case Study of the Czech Republic
- PMID: 32504401
- PMCID: PMC7704485
- DOI: 10.1007/s43441-020-00173-9
The Current Status of European and National Financial Sources for Clinical Research and Their Impact on Paediatric Non-commercial Clinical Trials: A Case Study of the Czech Republic
Abstract
Introduction: Paediatric non-commercial interventional clinical trials (NICTs) are crucial for healthcare provision. In spite of the fact that current regulations and initiatives try to enhance the quantity and quality of paediatric NICTs, there are still shortcomings that need to be addressed in order to accelerate the conduct of relevant clinical trials in children. To improve the current landscape of paediatric clinical research, it is necessary to identify and analyse the main trends and shortcomings, along with their impact on national performance in paediatric NICTs and this is the aim of this work.
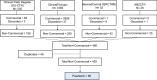
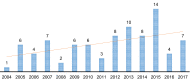
Method: A retrospective systematic search of paediatric NICTs was performed on four international clinical trials registries. Entries were filtered by date from 01/01/2004 to 31/12/2017. Each identified paediatric NICT was screened and analysed for sponsors, funders, type of intervention, therapeutic area, design characteristics and associated publications.
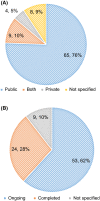
Results: The search identified 439 unique NICTs. When stratifying the trials by enrolment ages, 86 trials were found involving the paediatric population. Most trials investigated the use of medicinal products and were focused on cancer or cardiovascular diseases. The most common sources of the funding were non-profit organizations. Furthermore, from the total number of completed trials, only half of them already published their results.
Conclusion: The main shortcomings-specifically, ethical, methodological and, in particular, economic obstacles were identified. There is a continual need for greater support and collaboration between all major stakeholders including health policymakers, grant agencies, research institutions, pharmaceutical industries and healthcare providers at the national and international level.
Keywords: Financial sources; Infrastructure; International Clinical Trials Registries; Non-commercial Clinical Trials; Paediatric clinical research.
Conflict of interest statement
All named authors have no conflict of interest to declare.
Figures
References
-
- Convention on the Rights of the Child. UN General Assembly. 1989. https://www.refworld.org/docid/3ae6b38f0.html. Accessed 9 May 2020
-
- Hawcutt DB, Cooney L, Oni L, Pirmohamed M. Precision dosing in children. Expert Rev Precis Med Drug Dev. 2016;1:69–78. doi: 10.1080/23808993.2016.1138845. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

