Inducible deletion of skeletal muscle AMPKα reveals that AMPK is required for nucleotide balance but dispensable for muscle glucose uptake and fat oxidation during exercise
- PMID: 32504885
- PMCID: PMC7356270
- DOI: 10.1016/j.molmet.2020.101028
Inducible deletion of skeletal muscle AMPKα reveals that AMPK is required for nucleotide balance but dispensable for muscle glucose uptake and fat oxidation during exercise
Abstract
Objective: Evidence for AMP-activated protein kinase (AMPK)-mediated regulation of skeletal muscle metabolism during exercise is mainly based on transgenic mouse models with chronic (lifelong) disruption of AMPK function. Findings based on such models are potentially biased by secondary effects related to a chronic lack of AMPK function. To study the direct effect(s) of AMPK on muscle metabolism during exercise, we generated a new mouse model with inducible muscle-specific deletion of AMPKα catalytic subunits in adult mice.
Methods: Tamoxifen-inducible and muscle-specific AMPKα1/α2 double KO mice (AMPKα imdKO) were generated by using the Cre/loxP system, with the Cre under the control of the human skeletal muscle actin (HSA) promoter.
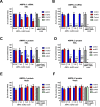
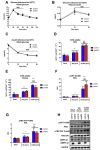
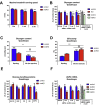
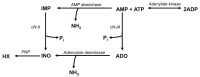
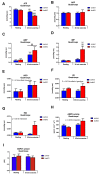
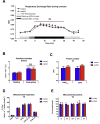
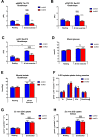
Results: During treadmill running at the same relative exercise intensity, AMPKα imdKO mice showed greater depletion of muscle ATP, which was associated with accumulation of the deamination product IMP. Muscle-specific deletion of AMPKα in adult mice promptly reduced maximal running speed and muscle glycogen content and was associated with reduced expression of UGP2, a key component of the glycogen synthesis pathway. Muscle mitochondrial respiration, whole-body substrate utilization, and muscle glucose uptake and fatty acid (FA) oxidation during muscle contractile activity remained unaffected by muscle-specific deletion of AMPKα subunits in adult mice.
Conclusions: Inducible deletion of AMPKα subunits in adult mice reveals that AMPK is required for maintaining muscle ATP levels and nucleotide balance during exercise but is dispensable for regulating muscle glucose uptake, FA oxidation, and substrate utilization during exercise.
Keywords: AMPK; Exercise; Fat oxidation; Glucose uptake; Glycogen; Muscle metabolism.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures
References
-
- Holloszy J.O., Kohrt W.M. Regulation of carbohydrate and fat metabolism during and after exercise. Annual Review of Nutrition. 1996;16:121–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

