The Underlying Mechanisms of Noncoding RNAs in the Chemoresistance of Hepatocellular Carcinoma
- PMID: 32505000
- PMCID: PMC7270498
- DOI: 10.1016/j.omtn.2020.05.011
The Underlying Mechanisms of Noncoding RNAs in the Chemoresistance of Hepatocellular Carcinoma
Abstract
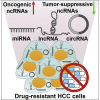
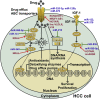
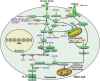
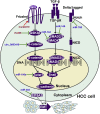
Hepatocellular carcinoma (HCC) is one of the most lethal human malignancies. Chemotherapeutic agents, such as sorafenib and lenvatinib, can improve the outcomes of HCC patients. Nevertheless, chemoresistance has become a major hurdle in the effective treatment of HCC. Noncoding RNAs (ncRNAs), including mircoRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs), have been demonstrated to participate in the onset and progression of HCC. Moreover, multiple lines of evidence have indicated that ncRNAs also play a pivotal role in HCC drug resistance. ncRNAs can regulate drug efflux and metabolism, glucose metabolism, cellular death pathways, and malignant characteristics in HCC. A deeper understanding of the molecular mechanisms responsible for ncRNA-mediated drug resistance in HCC will provide new opportunities for improving the treatment of HCC. In this review, we summarize recent findings on the molecular mechanisms by which ncRNAs regulate HCC chemoresistance, as well as their potential clinical implications in overcoming HCC chemoresistance.
Keywords: chemoresistance; circular RNAs; hepatocellular carcinoma; long noncoding RNAs; microRNAs.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The Role of Non-Coding RNAs in the Sorafenib Resistance of Hepatocellular Carcinoma.Front Oncol. 2021 Jul 22;11:696705. doi: 10.3389/fonc.2021.696705. eCollection 2021. Front Oncol. 2021. PMID: 34367979 Free PMC article. Review.
-
Non-coding RNA in drug resistance of hepatocellular carcinoma.Biosci Rep. 2018 Oct 9;38(5):BSR20180915. doi: 10.1042/BSR20180915. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30224380 Free PMC article. Review.
-
The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma.Mol Cancer. 2019 Oct 25;18(1):147. doi: 10.1186/s12943-019-1086-z. Mol Cancer. 2019. PMID: 31651347 Free PMC article. Review.
-
Noncoding RNAs in osteosarcoma: Implications for drug resistance.Cancer Lett. 2021 Apr 28;504:91-103. doi: 10.1016/j.canlet.2021.02.007. Epub 2021 Feb 12. Cancer Lett. 2021. PMID: 33587978
-
Noncoding RNAs in triple negative breast cancer: Mechanisms for chemoresistance.Cancer Lett. 2021 Dec 28;523:100-110. doi: 10.1016/j.canlet.2021.09.038. Epub 2021 Sep 30. Cancer Lett. 2021. PMID: 34601022
Cited by
-
The Role of Non-Coding RNAs in the Sorafenib Resistance of Hepatocellular Carcinoma.Front Oncol. 2021 Jul 22;11:696705. doi: 10.3389/fonc.2021.696705. eCollection 2021. Front Oncol. 2021. PMID: 34367979 Free PMC article. Review.
-
Aurora-A promotes lenvatinib resistance experimentally through hsa-circ-0058046/miR-424-5p/FGFR1 axis in hepatocellular carcinoma.Int J Immunopathol Pharmacol. 2025 Jan-Dec;39:3946320251316692. doi: 10.1177/03946320251316692. Int J Immunopathol Pharmacol. 2025. PMID: 39895095 Free PMC article.
-
A curcumin analog GL63 inhibits the malignant behaviors of hepatocellular carcinoma by inactivating the JAK2/STAT3 signaling pathway via the circular RNA zinc finger protein 83/microRNA-324-5p/cyclin-dependent kinase 16 axis.J Gastroenterol Hepatol. 2021 Oct;36(10):2967-2977. doi: 10.1111/jgh.15545. Epub 2021 Jun 3. J Gastroenterol Hepatol. 2021. PMID: 33982329 Free PMC article.
-
Lnc-SNHG5 Promoted Hepatocellular Carcinoma Progression Through the RPS3-NFκB Pathway.Int J Gen Med. 2023 Dec 1;16:5651-5664. doi: 10.2147/IJGM.S442937. eCollection 2023. Int J Gen Med. 2023. PMID: 38059157 Free PMC article.
-
LRGCPND: Predicting Associations between ncRNA and Drug Resistance via Linear Residual Graph Convolution.Int J Mol Sci. 2021 Sep 29;22(19):10508. doi: 10.3390/ijms221910508. Int J Mol Sci. 2021. PMID: 34638849 Free PMC article.
References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. - PubMed
-
- Villanueva A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019;380:1450–1462. - PubMed
-
- Llovet J.M., Montal R., Sia D., Finn R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018;15:599–616. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

