GCaMP as an indirect measure of electrical activity in rat trigeminal ganglion neurons
- PMID: 32505783
- PMCID: PMC7331800
- DOI: 10.1016/j.ceca.2020.102225
GCaMP as an indirect measure of electrical activity in rat trigeminal ganglion neurons
Abstract
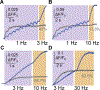
While debate continues over whether somatosensory information is transmitted via labeled line, population coding, frequency coding, or some combination therein, researchers have begun to address this question at the level of the primary afferent by using optical approaches that enable the assessment of neural activity in hundreds to even thousands of neurons simultaneously. However, with limited availability of tools to optically assess electrical activity in large populations of neurons, researchers have turned to genetically encoded Ca2+ indicators (GECIs) including GCaMP to enable the detection of increases in cytosolic Ca2+ concentrations as a correlate for neuronal activity. One of the most widely used GECIs is GCaMP6, which is available in three different versions tuned for sensitivity (GCaMP6s), speed (GCaMP6f), or a balance of the two (GCaMP6m). In order to determine if these issues were unique to GCaMP6 itself, or if they were inherent to more than one generation of GCaMP, we also characterized jGCaMP7. In the present study, we sought to determine the utility of the three GCaMP6 isoforms to detect changes in activity in primary afferents at frequencies ranging from 0.1-30 Hz. Given the heterogeneity of sensory neurons, we also compared the performance of each GCaMP6 isoform in subpopulations of neurons defined by properties used to identify putative nociceptive afferents: cell body size, isolectin B4 (IB4) binding, and capsaicin sensitivity. Finally, we compared results generated with GCaMP6 with that generated from neurons expressing the next generation of GCaMP, jGCaMP7s and jGCaMP7f. A viral approach, with AAV9-CAG-GCaMP6s/m/f, was used to drive GECI expression in acutely dissociated rat trigeminal ganglion (TG) neurons, and neural activity was driven by electrical field stimulation. Infection efficiency with the AAV serotype was high >95 %, and the impact of GCaMP6 expression in TG neurons over the period of study (<10 days) on the regulation of intracellular Ca2+, as assessed with fura-2, was minimal. Having confirmed that the field stimulation evoked Ca2+ transients were dependent on Ca2+ influx secondary to the activation of action potentials and voltage-gated Ca2+ channels, we also confirmed that the signal-to-noise ratio for each of the isoforms was excellent, enabling detection of a single spike in>90% of neurons. However, the utility of the GCaMP6 isoforms to enable an assessment of the firing frequency let alone changes in firing frequency of each neuron was relatively limited and isoform specific: GCaMP6s and 6m had the lowest resolution, enabling detection of spikes at 3 Hz in 15% and 32% of neurons respectively, but it was possible to resolve discrete single spikes up to 10 Hz in 36% of GCaMP6f neurons. Unfortunately, using other parameters of the Ca2+ transient, such as magnitude of the transient or the rate of rise, did not improve the range over which these indicators could be used to assess changes in spike number or firing frequency. Furthermore, in the presence of ongoing neural activity, it was even more difficult to detect a change in firing frequency. The frequency response relationship for the increase in Ca2+ was highly heterogeneous among sensory neurons and was influenced by both the GCaMP6 isoform used to assess it, the timing between the delivery of stimulation trains (inter-burst interval), and afferent subpopulation. Notably, the same deficiencies were observed with jGCaMP7s and 7f in resolving the degree of activity as were present for the GCaMP6 isoforms. Together, these data suggest that while both GCaMP6 and jGCaMP7 are potentially useful tools in sensory neurons to determine the presence or absence of neural activity, the ability to discriminate changes in firing frequency ≥ 3 Hz is extremely limited. As a result, GECIs should probably not be used in sensory neurons to assess changes in activity within or between subpopulations of neurons.
Keywords: Ca(2+)imaging; Intracellular calcium; Primary afferent; Sensory neurons; Somatosensory coding.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that there are no conflicts of interest.
Figures
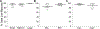


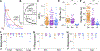
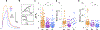
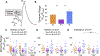


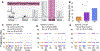
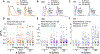
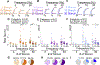

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

