Improving Oral Health and Modulating the Oral Microbiome to Reduce Bloodstream Infections from Oral Organisms in Pediatric and Young Adult Hematopoietic Stem Cell Transplantation Recipients: A Randomized Controlled Trial
- PMID: 32505810
- PMCID: PMC11168732
- DOI: 10.1016/j.bbmt.2020.05.019
Improving Oral Health and Modulating the Oral Microbiome to Reduce Bloodstream Infections from Oral Organisms in Pediatric and Young Adult Hematopoietic Stem Cell Transplantation Recipients: A Randomized Controlled Trial
Abstract
Bloodstream infections (BSIs) from oral organisms are a significant cause of morbidity and mortality in hematopoietic stem cell transplantation (HSCT) recipients. There are no proven strategies to decrease BSIs from oral organisms. The aim of this study was to evaluate the impact of daily xylitol wipes in improving oral health, decreasing BSI from oral organisms, and modulating the oral microbiome in pediatric HSCT recipients. This was a single-center 1:1 randomized controlled trial in pediatric HSCT recipients age >2 years. Age-matched healthy children were enrolled to compare the oral microbiome. The oral hygiene standard of care (SOC) group continued to receive the standard oral hygiene regimen. The xylitol group received daily oral xylitol wipes (with .7 g xylitol) in addition to the SOC. The intervention started from the beginning of the transplantation chemotherapy regimen and extended to 28 days following transplantation. The primary outcome was oral health at interval time points, and secondary outcomes included BSIs from oral organisms in the first 30 days following transplantation, oral microbiome abundance, and diversity and oral pathogenic organism abundance. The study was closed early due to efficacy after an interim analysis of the first 30 HSCT recipients was performed (SOC group, n = 16; xylitol group, n = 14). The xylitol group had a significantly lower rate of gingivitis at days 7, 14, and 28 following transplantation (P = .031, .0039, and .0005, respectively); oral plaque at days 7 and 14 (P = .045 and .0023, respectively); and oral ulcers >10 mm at day 14 (P = .049) compared with the SOC group. The xylitol group had no BSI from oral organisms compared with the SOC group, which had 4 (P = .04). The xylitol group had significantly lower abundance of potential BSI pathogens, such as Staphylococcus aureus (P = .036), Klebsiella pneumoniae (P = .033), and Streptococcus spp (P = .011) at the day after transplantation compared with the SOC group. Healthy children and young adults had significantly increased oral microbiome diversity compared with all HSCT recipients (P < .001). The addition of xylitol to standard oral care significantly improves oral health, decreases BSI from oral organisms, and decreases the abundance of pathogenic oral organisms in pediatric and young adult HSCT recipients.
Keywords: Bloodstream infection; Hematopoietic stem cell transplantation; Microbiome; Mucosal barrier injury laboratory-confirmed bloodstream infection; Xylitol.
Copyright © 2020 American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
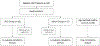
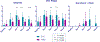

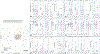
Similar articles
-
Bloodstream Infections and Colonization in Hematopoietic Stem Cell Transplant Recipients at a South African Center: A Retrospective Analysis.Transplant Cell Ther. 2025 Apr;31(4):269.e1-269.e13. doi: 10.1016/j.jtct.2025.02.010. Epub 2025 Feb 12. Transplant Cell Ther. 2025. PMID: 39952366
-
Gut Colonization Preceding Mucosal Barrier Injury Bloodstream Infection in Pediatric Hematopoietic Stem Cell Transplantation Recipients.Biol Blood Marrow Transplant. 2019 Nov;25(11):2274-2280. doi: 10.1016/j.bbmt.2019.07.019. Epub 2019 Jul 18. Biol Blood Marrow Transplant. 2019. PMID: 31326608 Free PMC article.
-
Changes in Vitamin D and Gut Microbiota in Pediatric Hematopoietic Stem Cell Transplantation Patients with Bloodstream Infections.Int J Vitam Nutr Res. 2025 Feb 12;95(1):26126. doi: 10.31083/IJVNR26126. Int J Vitam Nutr Res. 2025. PMID: 40134246
-
Safety and Efficacy of Prophylactic Levofloxacin in Pediatric and Adult Hematopoietic Stem Cell Transplantation Patients.Transplant Cell Ther. 2022 Mar;28(3):167.e1-167.e5. doi: 10.1016/j.jtct.2021.11.017. Epub 2021 Dec 4. Transplant Cell Ther. 2022. PMID: 34875405
-
Levofloxacin prophylaxis in pediatric oncology and hematopoietic stem cell transplantation: a literature review.Pediatr Hematol Oncol. 2024 Sep;41(6):432-448. doi: 10.1080/08880018.2024.2353888. Epub 2024 Jul 8. Pediatr Hematol Oncol. 2024. PMID: 38975680 Free PMC article. Review.
Cited by
-
Risk factors of bloodstream infection after allogeneic hematopoietic cell transplantation in children/adolescent and young adults.PLoS One. 2024 Aug 7;19(8):e0308395. doi: 10.1371/journal.pone.0308395. eCollection 2024. PLoS One. 2024. PMID: 39110739 Free PMC article.
-
The Impact of Human Microbiotas in Hematopoietic Stem Cell and Organ Transplantation.Front Immunol. 2022 Jul 7;13:932228. doi: 10.3389/fimmu.2022.932228. eCollection 2022. Front Immunol. 2022. PMID: 35874759 Free PMC article. Review.
-
Dental consensus on HSCT - Part II: dental Care during HSCT.Hematol Transfus Cell Ther. 2023 Jul-Sep;45(3):368-378. doi: 10.1016/j.htct.2023.04.003. Epub 2023 May 30. Hematol Transfus Cell Ther. 2023. PMID: 37321878 Free PMC article. Review.
-
Oral microbial changes and oral disease management before and after the treatment of hematological malignancies: a narrative review.Clin Oral Investig. 2023 Aug;27(8):4083-4106. doi: 10.1007/s00784-023-05021-2. Epub 2023 Apr 18. Clin Oral Investig. 2023. PMID: 37071220 Review.
-
Temporal variation in oral microbiome composition of patients undergoing autologous hematopoietic cell transplantation with keratinocyte growth factor.BMC Microbiol. 2023 Sep 13;23(1):258. doi: 10.1186/s12866-023-03000-x. BMC Microbiol. 2023. PMID: 37704974 Free PMC article.
References
-
- Barriga F, Ramírez P, Wietstruck A, Rojas N. Hematopoietic stem cell transplantation: clinical use and perspectives. Biol Res. 2012;45:307–316. - PubMed
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. - PubMed
-
- Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transplant. 2017;52:1091–1106. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

