Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop
- PMID: 32505910
- PMCID: PMC7834360
- DOI: 10.1016/j.mehy.2020.109906
Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop
Abstract
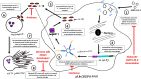
Most COVID-19 infected individuals present with mild flu-like symptoms; however, 5-10% of cases suffer from life-threatening pneumonia and respiratory failure. The pathogenesis of SARS-CoV-2 and its pathology of associated acute lung injury (ALI), acute respiratory distress syndrome (ARDS), sepsis, coagulopathy and multiorgan failure is not known. SARS-CoV-2 is an envelope virus with S (spike), M (membrane), N (nucleocapsid) and E (envelop) proteins. In a closely related coronavirus (SARS-CoV), the transmembrane E protein exerts an important role in membrane-ionic transport through viroporins, deletion of which reduced levels of IL-1β and a remarkably reduced lung edema compared to wild type. IL-1β is generated by macrophages upon activation of intracellular NLRP3 (NOD-like, leucine rich repeat domains, and pyrin domain-containing protein 3), part of the functional NLRP3 inflammasome complex that detects pathogenic microorganisms and stressors, while neutrophils are enhanced by increasing levels of IL-1β. Expiring neutrophils undergo "NETosis", producing thread-like extracellular structures termed neutrophil extracellular traps (NETs), which protect against mild infections and microbes. However, uncontrolled NET production can cause acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), coagulopathy, multiple organ failure, and autoimmune disease. Herein, we present arguments underlying our hypothesis that IL-1β and NETs, mediated via NLRP3 inflammasomes, form a feed-forward loop leading to the excessive alveolar and endothelial damage observed in severe cases of COVID-19. Considering such assertions, we propose potential drug candidates that could be used to alleviate such pathologies. Considering that recent efforts to ascertain effective treatments of COVID-19 in severe patients has been less than successful, investigating novel avenues of treating this virus are essential.
Keywords: COVID19; Coronavirus; Inflammasomes; Neutrophil extracellular traps (NETs); SARS.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Ahmed Yaqinuddin and Junaid Kashir declare that there is no conflict of interest either financial or personal relationships with other people or organisations that could inappropriately influence (bias) our work.
Figures
Similar articles
-
Targeting the NLRP3 Inflammasome in Severe COVID-19.Front Immunol. 2020 Jun 23;11:1518. doi: 10.3389/fimmu.2020.01518. eCollection 2020. Front Immunol. 2020. PMID: 32655582 Free PMC article. Review.
-
Neutrophil Extracellular Traps (NETs) and Covid-19: A new frontiers for therapeutic modality.Int Immunopharmacol. 2022 Mar;104:108516. doi: 10.1016/j.intimp.2021.108516. Epub 2022 Jan 6. Int Immunopharmacol. 2022. PMID: 35032828 Free PMC article. Review.
-
COVID-19: Role of neutrophil extracellular traps in acute lung injury.Respir Investig. 2020 Sep;58(5):419-420. doi: 10.1016/j.resinv.2020.06.001. Epub 2020 Jun 26. Respir Investig. 2020. PMID: 32611518 Free PMC article. No abstract available.
-
A novel cell-based assay for dynamically detecting neutrophil extracellular traps-induced lung epithelial injuries.Exp Cell Res. 2020 Sep 15;394(2):112101. doi: 10.1016/j.yexcr.2020.112101. Epub 2020 May 29. Exp Cell Res. 2020. PMID: 32474064 Free PMC article.
-
Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression.Adv Biol Regul. 2020 Aug;77:100741. doi: 10.1016/j.jbior.2020.100741. Epub 2020 Jul 4. Adv Biol Regul. 2020. PMID: 32773102 Free PMC article. Review.
Cited by
-
COVID-19: imbalanced cell-mediated immune response drives to immunopathology.Emerg Microbes Infect. 2022 Dec;11(1):2393-2404. doi: 10.1080/22221751.2022.2122579. Emerg Microbes Infect. 2022. PMID: 36069182 Free PMC article. Review.
-
Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage.Transl Oncol. 2021 Jul;14(7):101095. doi: 10.1016/j.tranon.2021.101095. Epub 2021 Apr 19. Transl Oncol. 2021. PMID: 33887552 Free PMC article. Review.
-
COVID-19: Immunology, Immunopathogenesis and Potential Therapies.Int Rev Immunol. 2022;41(2):171-206. doi: 10.1080/08830185.2021.1883600. Epub 2021 Feb 27. Int Rev Immunol. 2022. PMID: 33641587 Free PMC article. Review.
-
Cross-immunity between respiratory coronaviruses may limit COVID-19 fatalities.Med Hypotheses. 2020 Nov;144:110049. doi: 10.1016/j.mehy.2020.110049. Epub 2020 Jun 30. Med Hypotheses. 2020. PMID: 32758887 Free PMC article.
-
Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19.J Nanobiotechnology. 2021 Jun 10;19(1):173. doi: 10.1186/s12951-021-00926-0. J Nanobiotechnology. 2021. PMID: 34112203 Free PMC article.
References
-
- Fung T.S., Liu D.X. Human Coronavirus: host-pathogen interaction. Annu Rev Microbiol. 2019;73:529–557. Epub 2019/06/22. 10.1146/annurev-micro-020518-115759 PubMed PMID: 31226023. - PubMed
-
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. PubMed PMID: 31987001; PubMed Central PMCID: PMCPMC7067204. - DOI - PMC - PubMed
-
- Raamsman M.J., Locker J.K., de Hooge A., de Vries A.A., Griffiths G., Vennema H. Characterization of the Coronavirus mouse hepatitis virus strain A59 small membrane protein E. J Virol. 2000;74(5):2333–2342. doi: 10.1128/JVI.74.5.2333-2342.2000. PubMed PMID: 10666264; PubMed Central PMCID: PMCPMC111715. - DOI - PMC - PubMed
-
- DeDiego M.L., Alvarez E., Almazan F., Rejas M.T., Lamirande E., Roberts A. A severe acute respiratory syndrome Coronavirus that lacks the e gene is attenuated in vitro and in vivo. JVI. 2007;81(4):1701–1713. doi: 10.1128/JVI.01467-06. PubMed PMID: 17108030; PubMed Central PMCID: PMCPMC1797558. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

