Organophosphorus compounds and oximes: a critical review
- PMID: 32506210
- PMCID: PMC7367912
- DOI: 10.1007/s00204-020-02797-0
Organophosphorus compounds and oximes: a critical review
Abstract
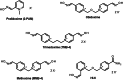
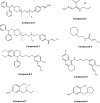
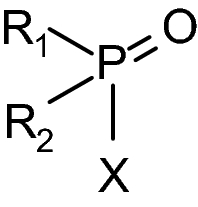
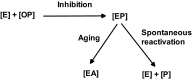
Organophosphorus (OP) pesticides and nerve agents still pose a threat to the population. Treatment of OP poisoning is an ongoing challenge and burden for medical services. Standard drug treatment consists of atropine and an oxime as reactivator of OP-inhibited acetylcholinesterase and is virtually unchanged since more than six decades. Established oximes, i.e. pralidoxime, obidoxime, TMB-4, HI-6 and MMB-4, are of insufficient effectiveness in some poisonings and often cover only a limited spectrum of the different nerve agents and pesticides. Moreover, the value of oximes in human OP pesticide poisoning is still disputed. Long-lasting research efforts resulted in the preparation of countless experimental oximes, and more recently non-oxime reactivators, intended to replace or supplement the established and licensed oximes. The progress of this development is slow and none of the novel compounds appears to be suitable for transfer into advanced development or into clinical use. This situation calls for a critical analysis of the value of oximes as mainstay of treatment as well as the potential and limitations of established and novel reactivators. Requirements for a straightforward identification of superior reactivators and their development to licensed drugs need to be addressed as well as options for interim solutions as a chance to improve the therapy of OP poisoning in a foreseeable time frame.
Keywords: Acetylcholinesterase; Nerve agents; Organophosphorus compounds; Oximes; Pesticides; Reactivation.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures
References
-
- Ashani Y, Bhattacharjee AK, Leader H, Saxena A, Doctor BP. Inhibition of cholinesterases with cationic phosphonyl oximes highlights distinctive properties of the charged pyridine groups of quaternary oxime reactivators. Biochem Pharmacol. 2003;66:191–202. - PubMed
-
- Aurbek N, Herkert NM, Koller M, Thiermann H, Worek F. Kinetic analysis of interactions of different sarin and tabun analogues with human acetylcholinesterase and oximes: Is there a structure-activity relationship? Chem Biol Interact. 2010;187:215–219. - PubMed
-
- Aurbek N, Thiermann H, Eyer F, Eyer P, Worek F. Suitability of human butyrylcholinesterase as therapeutic marker and pseudo catalytic scavenger in organophosphate poisoning: a kinetic analysis. Toxicology. 2009;259:133–139. - PubMed
-
- Aurbek N, Thiermann H, Szinicz L, Eyer P, Worek F. Analysis of inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds with human and pig acetylcholinesterase. Toxicology. 2006;224:91–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

