Erythropoietin prevents LPS-induced preterm birth and increases offspring survival
- PMID: 32506750
- PMCID: PMC7507205
- DOI: 10.1111/aji.13283
Erythropoietin prevents LPS-induced preterm birth and increases offspring survival
Abstract
Problem: Preterm delivery is the leading cause of neonatal mortality and contributes to delayed physical and cognitive development in children. At present, there is no efficient therapy to prevent preterm labor. A large body of evidence suggests that infections might play a significant and potentially preventable cause of premature birth. This work assessed the effects of erythropoietin (EPO) in a murine model of inflammation-associated preterm delivery, which mimics central features of preterm infections in humans.
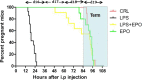
Method of study: BALB/c mice were injected i.p. with 20 000 IU/kg EPO or normal saline twice on gestational day (GD) 15, with a 3 hours time interval between injections. An hour after the first EPO or normal saline injection, all mice received two injections of 50 μg/kg LPS, also given 3 hours apart.
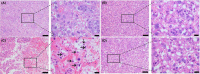
Results: EPO significantly prevented preterm labor and increased offspring survival in an LPS induced preterm delivery model. EPO prevented LPS-induced leukocyte infiltration into the placenta. Moreover, EPO inhibited the expression of pro-inflammatory cytokines, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-α (TNF-α) in maternal serum and amniotic fluid. EPO also prevented LPS-induced increase in placental prostaglandin (PG)E2 and uterine inducible nitric oxide synthase (iNOS) production, while decreasing nuclear factor kappa-B (NF-κβ) activity in the myometrium. EPO also increased the gene expression of placental programmed cell death ligand 1 (PD-L1) in LPS-treated mice.
Conclusions: Our results suggest that EPO could be a potential novel therapeutic strategy to tackle infection-related preterm labor.
Keywords: PDL1; erythropoietin; inflammation; offspring survival; preterm birth; prostaglandins.
© 2020 The Authors. American Journal of Reproductive Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Melatonin prevents experimental preterm labor and increases offspring survival.J Pineal Res. 2014 Mar;56(2):154-62. doi: 10.1111/jpi.12108. Epub 2014 Jan 2. J Pineal Res. 2014. PMID: 24313220
-
Resveratrol protects from lipopolysaccharide-induced inflammation in the uterus and prevents experimental preterm birth.Mol Hum Reprod. 2017 Aug 1;23(8):571-581. doi: 10.1093/molehr/gax036. Mol Hum Reprod. 2017. PMID: 28810692 Free PMC article.
-
Erythropoietin attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain.Neonatology. 2007;92(4):269-78. doi: 10.1159/000105493. Epub 2007 Jul 11. Neonatology. 2007. PMID: 17627093
-
Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice.PLoS One. 2013 Dec 6;8(12):e82713. doi: 10.1371/journal.pone.0082713. eCollection 2013. PLoS One. 2013. PMID: 24324824 Free PMC article.
-
Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model.Am J Obstet Gynecol. 2006 Dec;195(6):1578-89. doi: 10.1016/j.ajog.2006.06.072. Am J Obstet Gynecol. 2006. PMID: 17132473
Cited by
-
Harnessing stem cell therapeutics in LPS-induced animal models: mechanisms, efficacies, and future directions.Stem Cell Res Ther. 2025 Apr 12;16(1):176. doi: 10.1186/s13287-025-04290-w. Stem Cell Res Ther. 2025. PMID: 40221751 Free PMC article. Review.
-
Signaling Pathways Regulating Human Cervical Ripening in Preterm and Term Delivery.Cells. 2022 Nov 21;11(22):3690. doi: 10.3390/cells11223690. Cells. 2022. PMID: 36429118 Free PMC article. Review.
-
Pre-conditioning with PQQ during pregnancy alleviates LPS-induced placental damage and improves the fetal survival and growth in mice.Front Endocrinol (Lausanne). 2025 Jul 10;16:1617026. doi: 10.3389/fendo.2025.1617026. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40708725 Free PMC article.
-
Erythropoietin Improves Atrophy, Bleeding and Cognition in the Newborn Intraventricular Hemorrhage.Front Cell Dev Biol. 2020 Sep 16;8:571258. doi: 10.3389/fcell.2020.571258. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33043002 Free PMC article.
-
Link between TLR4/MyD88/NF-κB levels, inflammation, and infectious preterm birth in pregnant women.Medicine (Baltimore). 2025 Aug 8;104(32):e43509. doi: 10.1097/MD.0000000000043509. Medicine (Baltimore). 2025. PMID: 40797405 Free PMC article.
References
-
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162‐2172. - PubMed
-
- McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82‐90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

