Desensitizing highly sensitized heart transplant candidates with the combination of belatacept and proteasome inhibition
- PMID: 32506824
- PMCID: PMC8366746
- DOI: 10.1111/ajt.16113
Desensitizing highly sensitized heart transplant candidates with the combination of belatacept and proteasome inhibition
Abstract
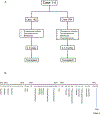
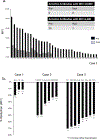
HLA antibodies pose a significant barrier to transplantation and current strategies to reduce allosensitization are limited. We hypothesized that augmenting proteasome inhibitor (PI) based desensitization with costimulation blockade (belatacept) to mitigate germinal center (GC) responses might increase efficacy and prevent rebound. Four highly sensitized (calculated panel reactive antibody [cPRA] class I and/or II >99%, complement-dependent cytotoxicity panel reactive antibody [CDC PRA+], C1q+) heart transplant candidates were treated with the combination of belatacept and PI therapy, which significantly reduced both class I and II HLA antibodies and increased the likelihood of identifying an acceptable donor. Three negative CDC crossmatches were achieved against 3, 6, and 8 donor-specific antibodies (DSA), including those that were historically C1q+ binding. Posttransplant, sustained suppression of 3 of 3, 4 of 6, and 8 of 8 DSA (cases 1-3) was achieved. Analysis of peripheral blood mononuclear cells before and after desensitization in one case revealed a decrease in naïve and memory B cells and a reduction in T follicular helper cells with a phenotype suggesting recent GC activity (CD38, PD1, and ICOS). Furthermore, a shift in the natural killer cell phenotype was observed with features suggestive of activation. Our findings support synergism between PI based desensitization and belatacept facilitating transplantation with a negative CDC crossmatch against historically strong, C1q binding antibodies.
Keywords: costimulation; desensitization; heart transplantation/cardiology; histocompatibility; immunosuppressant-fusion proteins and monoclonal antibodies: belatacept; immunosuppression/immune modulation; natural killer (NK) cells/NK receptors; sensitization; translational research/science.
© 2020 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures



References
-
- Colvin MM, Cook JL, Chang PP, et al.Sensitization in Heart Transplantation: Emerging Knowledge: A Scientific Statement From the American Heart Association. Circulation. 2019;139(12): e553–e578. - PubMed
-
- Kransdorf EP, Kittleson MM, Patel JK, Pando MJ, Steidley DE, Kobashigawa JA. Calculated panel-reactive antibody predicts outcomes on the heart transplant waiting list. J Heart Lung Transplant. 2017;36(7): 787–796. - PubMed
-
- Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant. 2011;30(12): 1320–1326. - PubMed
-
- Jordan SC, Lorant T, Choi J, et al.IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N Engl J Med. 2017;377(5): 442–453. - PubMed
-
- Vo AA, Lukovsky M, Toyoda M, et al.Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3): 242–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

