Omega-3 Polyunsaturated Fatty Acids Decrease Aortic Valve Disease Through the Resolvin E1 and ChemR23 Axis
- PMID: 32506925
- PMCID: PMC7439935
- DOI: 10.1161/CIRCULATIONAHA.119.041868
Omega-3 Polyunsaturated Fatty Acids Decrease Aortic Valve Disease Through the Resolvin E1 and ChemR23 Axis
Abstract
Background: Aortic valve stenosis (AVS), which is the most common valvular heart disease, causes a progressive narrowing of the aortic valve as a consequence of thickening and calcification of the aortic valve leaflets. The beneficial effects of omega-3 polyunsaturated fatty acids (n-3 PUFAs) in cardiovascular prevention have recently been demonstrated in a large randomized, controlled trial. In addition, n-3 PUFAs serve as the substrate for the synthesis of specialized proresolving mediators, which are known by their potent beneficial anti-inflammatory, proresolving, and tissue-modifying properties in cardiovascular disease. However, the effects of n-3 PUFA and specialized proresolving mediators on AVS have not yet been determined. The aim of this study was to identify the role of n-3 PUFA-derived specialized proresolving mediators in relation to the development of AVS.
Methods: Lipidomic and transcriptomic analyses were performed in human tricuspid aortic valves. Apoe-/- mice and wire injury in C57BL/6J mice were used as models for mechanistic studies.
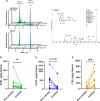
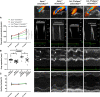
Results: We found that n-3 PUFA incorporation into human stenotic aortic valves was higher in noncalcified regions compared with calcified regions. Liquid chromatography tandem mass spectrometry-based lipid mediator lipidomics identified that the n-3 PUFA-derived specialized proresolving mediator resolvin E1 was dysregulated in calcified regions and acted as a calcification inhibitor. Apoe-/- mice expressing the Caenorhabditis elegans Fat-1 transgene (Fat-1tg×Apoe-/-), which enables the endogenous synthesis of n-3 PUFA and increased valvular n-3 PUFA content, exhibited reduced valve calcification, lower aortic valve leaflet area, increased M2 macrophage polarization, and improved echocardiographic parameters. Finally, abrogation of the resolvin E1 receptor ChemR23 enhanced disease progression, and the beneficial effects of Fat-1tg were abolished in the absence of ChemR23.
Conclusions: n-3 PUFA-derived resolvin E1 and its receptor ChemR23 emerge as a key axis in the inhibition of AVS progression and may represent a novel potential therapeutic opportunity to be evaluated in patients with AVS.
Keywords: calcification, physiologic; fatty acids, omega-3; heart valve diseases; inflammation; lipids.
Figures





References
-
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J. 1987; 8:471–483. doi: 10.1093/oxfordjournals.eurheartj.a062307 - PubMed
-
- Al-Azizi K, Hamandi M, Mack M. Clinical trials of transcatheter aortic valve replacement. Heart. 2019; 105suppl 2s6–s9. doi: 10.1136/heartjnl-2018-313511 - PubMed
-
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. ; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019; 380:11–22. doi: 10.1056/NEJMoa1812792 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

