Cutaneous barrier dysfunction in allergic diseases
- PMID: 32507227
- PMCID: PMC7291847
- DOI: 10.1016/j.jaci.2020.02.021
Cutaneous barrier dysfunction in allergic diseases
Erratum in
-
Corrigenda.J Allergy Clin Immunol. 2021 Sep;148(3):905. doi: 10.1016/j.jaci.2021.06.014. J Allergy Clin Immunol. 2021. PMID: 34489014 No abstract available.
Abstract
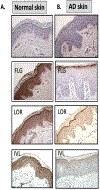
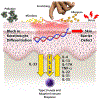
The fundamental defect(s) that drives atopic dermatitis (AD) remains controversial. "Outside in" proponents point to the important association of filaggrin gene mutations and other skin barrier defects with AD. The "inside out" proponents derive support from evidence that AD occurs in genetic animal models with overexpression of type 2 immune pathways in their skin, and humans with gain-of-function mutations in their type 2 response develop severe AD. The observation that therapeutic biologics, targeting type 2 immune responses, can reverse AD provides compelling support for the importance of "inside out" mechanisms of AD. In this review, we propose a central role for epithelial cell dysfunction that accounts for the dual role of skin barrier defects and immune pathway activation in AD. The complexity of AD has its roots in the dysfunction of the epithelial barrier that allows the penetration of allergens, irritants, and microbes into a cutaneous milieu that facilitates the induction of type 2 immune responses. The AD phenotypes and endotypes that result in chronic skin inflammation and barrier dysfunction are modified by genes, innate/adaptive immune responses, and different environmental factors that cause skin barrier dysfunction. There is also compelling evidence that skin barrier dysfunction can alter the course of childhood asthma, food allergy, and allergic rhinosinusitis. Effective management of AD requires a multipronged approach, not only restoring cutaneous barrier function, microbial flora, and immune homeostasis but also enhancing skin epithelial differentiation.
Keywords: Atopic dermatitis; epithelial barrier; food allergy; peanut allergy; skin barrier.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol 2017;140:145–53. DOI: 10.1016/j.jaci.2017.02.019. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

