External Validation of Model-Based Dosing Guidelines for Vancomycin, Gentamicin, and Tobramycin in Critically Ill Neonates and Children: A Pragmatic Two-Center Study
- PMID: 32507958
- PMCID: PMC7383037
- DOI: 10.1007/s40272-020-00400-8
External Validation of Model-Based Dosing Guidelines for Vancomycin, Gentamicin, and Tobramycin in Critically Ill Neonates and Children: A Pragmatic Two-Center Study
Abstract
Background: The Dutch Pediatric Formulary (DPF) increasingly bases its guidelines on model-based dosing simulations from pharmacokinetic studies. This resulted in nationwide dose changes for vancomycin, gentamicin, and tobramycin in 2015.
Objective: We aimed to evaluate target attainment of these altered, model-based doses in critically ill neonates and children.
Methods: This was a retrospective cohort study in neonatal intensive care unit (NICU) and pediatric ICU (PICU) patients receiving vancomycin, gentamicin, or tobramycin between January 2015 and March 2017 in two university hospitals. The first therapeutic drug monitoring concentration for each patient was collected, as was clinical and dosing information. Vancomycin and tobramycin target trough concentrations were 10-15 and ≤ 1 mg/L, respectively. Target gentamicin trough and peak concentrations were < 1 and 8-12 mg/L, respectively.
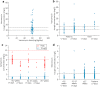
Results: In total, 482 patients were included (vancomycin [PICU] n = 62, [NICU] n = 102; gentamicin [NICU] n = 97; tobramycin [NICU] n = 221). Overall, median trough concentrations were within the target range for all cohorts but showed large interindividual variability, causing nontarget attainment. Trough concentrations were outside the target range in 66.1%, 60.8%, 14.7%, and 23.1% of patients in these four cohorts, respectively. Gentamicin peak concentrations were outside the range in 69% of NICU patients (term neonates 87.1%, preterm infants 57.1%). Higher creatinine concentrations were associated with higher vancomycin and tobramycin trough concentrations.
Conclusion: This study illustrates the need to validate model-based dosing advice in the real-world setting as both sub- and supratherapeutic concentrations of vancomycin, gentamicin, and tobramycin were very prevalent. Our data underline the necessity for further individualization by addressing the high interindividual variability to improve target attainment.
Conflict of interest statement
Prof. Dr. de Wildt is Director of the Dutch Pediatric Knowledge Center Pharmacotherapy for Children and as such is responsible for the Dutch Pediatric Formularies and its international editions. Stan J.F. Hartman, Lynn B. Orriëns, Samanta M. Zwaag, Tim Poel, and Marika de Hoop have no conflicts of interest that are directly relevant to the content of this article.
Figures

Comment in
-
Target Drug Exposure Attainment in Children: How to Get from Better to Best.Paediatr Drugs. 2020 Aug;22(4):445-447. doi: 10.1007/s40272-020-00402-6. Paediatr Drugs. 2020. PMID: 32507957 Free PMC article. No abstract available.
References
-
- van der Zanden TM, de Wildt SN, Liem Y, Offringa M, de Hoog M, Dutch Paediatric Pharmacotherapy Expertise Network N Developing a paediatric drug formulary for the Netherlands. Arch Dis Child. 2017;102(4):357–361. - PubMed
-
- De Cock RF, Allegaert K, Brussee JM, Sherwin CM, Mulla H, de Hoog M, et al. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration. Pharm Res. 2014;31(10):2643–2654. - PMC - PubMed
-
- Gross JR, Kaplan SL, Kramer WG, Mason EO., Jr Vancomycin pharmacokinetics in premature infants. Pediatr Pharmacol (NY) 1985;5(1):17–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

