Micro-environmental cross-talk in an organotypic human melanoma-in-skin model directs M2-like monocyte differentiation via IL-10
- PMID: 32507967
- PMCID: PMC7568725
- DOI: 10.1007/s00262-020-02626-4
Micro-environmental cross-talk in an organotypic human melanoma-in-skin model directs M2-like monocyte differentiation via IL-10
Abstract
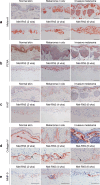
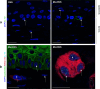
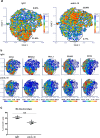
Preclinical assessment of novel therapies to fight cancer requires models that reflect the human physiology and immune response. Here, we established an in vitro three-dimensional (3D) reconstructed organotypic human melanoma-in-skin (Mel-RhS) model to investigate cellular and molecular features of tumor formation over a period of 6 weeks. Tumor nests developed over time at the epidermal-dermal junction and spread towards the dermis, in places disrupting the basement membrane. This coincided with secretion of matrix metalloproteinase 9 (MMP-9) by melanoma cells. These features resemble the initial stages of invasive melanoma. Interestingly, while the SK-MEL-28 cell line did not secrete detectable levels of interleukin-10 (IL-10) in traditional two-dimensional monolayers, it did express IL-10 in the 3D Mel-RhS, as did the surrounding keratinocytes and fibroblasts. This cellular cross-talk-induced secretion of IL-10 in the Mel-RhS indicated the generation of an immune suppressive microenvironment. Culture supernatants from Mel-RhS interfered with monocyte-to-dendritic-cell differentiation, leading to the development of M2-like macrophages, which was in part prevented by antibody-mediated IL-10 blockade. Indeed, high-dimensional single-cell analysis revealed a shift within the monocyte population away from a CD163+PD-L1+ M2-like phenotype upon IL-10 blockade. Thus, the 3D configuration of the Mel-RhS model revealed a role for IL-10 in immune escape through misdirected myeloid differentiation, which would have been missed in classical monolayer cultures.
Keywords: IL-10; M2 macrophages; Melanoma; Reconstructed human skin; Tumor microenvironment; Tumor progression.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
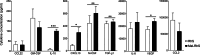

References
-
- Lindenberg JJ, van de Ven R, Lougheed SM, Zomer A, Santegoets SJAM, Griffioen AW, Hooijberg E, van den Eertwegh AJM, Thijssen VL, Scheper RJ, Oosterhoff D, de Gruijl TD. Functional characterization of a STAT3-dependent dendritic cell-derived CD14+ cell population arising upon IL-10-driven maturation. Oncoimmunology. 2013;2(4):e23837. doi: 10.4161/onci.23837. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

