Can routine laboratory tests discriminate SARS-CoV-2-infected pneumonia from other causes of community-acquired pneumonia?
- PMID: 32508038
- PMCID: PMC7274074
- DOI: 10.1002/ctm2.23
Can routine laboratory tests discriminate SARS-CoV-2-infected pneumonia from other causes of community-acquired pneumonia?
Abstract
Background: The clinical presentation of SARS-CoV-2-infected pneumonia (COVID-19) resembles that of other etiologies of community-acquired pneumonia (CAP). We aimed to identify clinical laboratory features to distinguish COVID-19 from CAP.
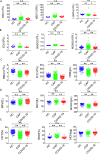
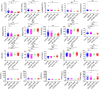
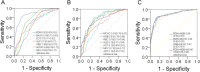
Methods: We compared the hematological and biochemical features of 84 patients with COVID-19 at hospital admission and 221 patients with CAP. Parameters independently predictive of COVID-19 were calculated by multivariate logistic regression. The receiver operating characteristic (ROC) curves were generated and the area under the ROC curve (AUC) was measured to evaluate the discriminative ability.
Results: Most hematological and biochemical indexes of patients with COVID-19 were significantly different from patients with CAP. Nine laboratory parameters were identified to be predictive of a diagnosis of COVID-19. The AUCs demonstrated good discriminatory ability for red cell distribution width (RDW) with an AUC of 0.87 and hemoglobin with an AUC of 0.81. Red blood cell, albumin, eosinophil, hematocrit, alkaline phosphatase, and mean platelet volume had fair discriminatory ability. Combinations of any two parameters performed better than did the RDW alone.
Conclusions: Routine laboratory examinations may be helpful for the diagnosis of COVID-19. Application of laboratory tests may help to optimize the use of isolation rooms for patients when they present with unexplained febrile respiratory illnesses.
Keywords: 2019 novel coronavirus; COVID-19; SARS-CoV-2; community-acquired pneumonia; routine laboratory tests.
© 2020 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- WHO . Coronavirus disease 2019 (COVID‐19). Situation Report‐62. 2020. Released on March 21, 2020. https://wwwwhoint/docs/default-source/coronaviruse/situation-reports/202...
-
- Stop the Wuhan virus. Nature. 2020;577(7791):450. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
