GDNF and Parkinson's Disease: Where Next? A Summary from a Recent Workshop
- PMID: 32508331
- PMCID: PMC7458523
- DOI: 10.3233/JPD-202004
GDNF and Parkinson's Disease: Where Next? A Summary from a Recent Workshop
Abstract
The concept of repairing the brain with growth factors has been pursued for many years in a variety of neurodegenerative diseases including primarily Parkinson's disease (PD) using glial cell line-derived neurotrophic factor (GDNF). This neurotrophic factor was discovered in 1993 and shown to have selective effects on promoting survival and regeneration of certain populations of neurons including the dopaminergic nigrostriatal pathway. These observations led to a series of clinical trials in PD patients including using infusions or gene delivery of GDNF or the related growth factor, neurturin (NRTN). Initial studies, some of which were open label, suggested that this approach could be of value in PD when the agent was injected into the putamen rather than the cerebral ventricles. In subsequent double-blind, placebo-controlled trials, the most recent reporting in 2019, treatment with GDNF did not achieve its primary end point. As a result, there has been uncertainty as to whether GDNF (and by extrapolation, related GDNF family neurotrophic factors) has merit in the future treatment of PD. To critically appraise the existing work and its future, a special workshop was held to discuss and debate this issue. This paper is a summary of that meeting with recommendations on whether there is a future for this therapeutic approach and also what any future PD trial involving GDNF and other GDNF family neurotrophic factors should consider in its design.
Keywords: GDNF; NRTN; Parkinson’s disease; clinical trials; dopaminergic neurons.
Conflict of interest statement
Roger Barker is a member of the advisory board for Novo Nordisk; Living Cell Technologies; FCDI; BlueRock Therapeutics, Aspen Neuroscience and UCB. He receives royalties from Springer-Nature and Wiley. He has received grant support from the EU; NIHR; MRC; Wellcome Trust; HDA; Rosetrees Trust; Cure Parkinson’s Trust; Parkinson’s UK and Evelyn Trust. He is Co-Editor in Chief of the Journal of Neurology and an Associate Editor for the Journal of Parkinson’s Disease.
Don Gash is a founding Partner in Avast Therapeutics Inc. with patents on drug candidates for treating neurological disorders.
Alan Whone was a PI on the recent UK GDNF trial that received support from Parkinson’s UK and the Cure Parkinson’s Trust
Anthony Lang reports consultancy support from Abbvie, Acorda, AFFiRis, Biogen, Denali, Janssen, Intracellular, Kallyope, Lundbeck, Paladin, Retrophin, Roche, Sun Pharma, Theravance, and Corticobasal Degeneration Solutions; advisory board support form Jazz Pharma, PhotoPharmics, Sunovion; other honoraria from Sun Pharma, AbbVie, Sunovion, American Academy of Neurology and the International Parkinson and Movement Disorder Society; grants from Brain Canada, Canadian Institutes of Health Research, Corticobasal Degeneration Solutions, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, Parkinson Foundation, Parkinson Canada, and W. Garfield Weston Foundation and royalties from Elsevier, Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press.
Amber van Laar is an employee of Brain Neurotherapy Bio, Inc.
Adrian Kells is an employee and stock holder of Brain Neurotherapy Bio, Inc.
Massimo Fiandaca is co-founder and Vice President, Clinical Affairs at Brain Neurotherapy Bio, Inc. He has an equity interest in this company.
Jeff Kordower serves as a consultant and scientific advisor to Fuji-Cellular dynamics Inc., a company performing preclinical, and is in the planning stages of clinical IPSC dopamine grafts in patients with PD. Dr. Kordower is also a consultant for Seelos- Inc., NsGene Inc., Brainstorm Inc., Abbvie, Inc., Biogen Inc., Brain Neurotherapy Bio, Clintrex Inc., Encora Inc., and Inhibikase Inc. Dr. Kordower is also an Associate Editor for Journal of Comparative Neurology, Neurobiology of Aging, and Journal of Parkinson’s Disease.
Kris Bankiewicz is the Founder and CEO of Brain Neurotherapy Bio that is developing AAV2-GDNF for PD, co-founder of Voyager Therapeutics that is developing AAV2AADC for PD, co-founder of MedGenesis Therapeutics that is developing rec. GDNF protein for PD and also has financial interest in all the companies.
Karl Kieburtz is a consultant for Clintrex Research Corp, Roche/Genentech, Novartis, Blackfynn LLC. He has grant support from NIH (NINDS, NCATS), Michael J Fox Foundation and has ownership inClintrex Research Corp, Hoover Brown LLC, Blackfynn LLC, Safe Therapeutics LLC.
Sigrid Booms is an employee of Herantis Pharma Plc.
Henri Huttunen is co-founder, shareholder and an employee of Herantis Pharma Plc.
A. Jon Stoessl reports personal fees from AbbVie, personal fees from Voyager/Neurocrine Biosciences, and is Editor-in-Chief, Movement Disorders (stipend).
Howard Federoff is a co-founder of Med Genesis Therapetix and Brain Neurotherapy Bio. He also serves as the CEO of Aspen Neuroscience. He is a consultant for Juvenescence, a scientific advisory board member for Ovid Therapeutics, chair of the board for Souvien and director for Perthera. He holds equity interests in all of the above.
Mart Saarma is founder and shareholder in the Herantis Pharma Plc. This company is testing CDNF in clinical trials.
Merja H. Voutilainen is an inventor in the CDNF-patent, which is owned by Herantis Pharma Plc.
Codrin Lungu has received honoraria from Elsevier, Inc. for editorial work. The work has been conducted as part of official duty in the course of employment with the NIH, and agency of the US Federal Government.
Anders Björklund, David Eidelberg, David Dexter, Jamie Eberling, Richard Wyse, Simon Stott, Eros Bresolin, Lyndsey Issacs, Patrik Brundin, Brain Fiske, Camille Carroll, Alasdair Coles, Leah Mursaleen all have no disclosures.
Patrik Brundin has received commercial support as a consultant from Renovo Neural, Inc., Lundbeck A/S, AbbVie, Fujifilm-Cellular Dynamics International, Axial Biotherapeutics, CuraSen, Idorsia Pharmaceuticals Ltd and Living Cell Technologies. He has received commercial support for research from Lundbeck A/S and Roche. He has ownership interests in Acousort AB and Axial Biotherapeutics.
Figures
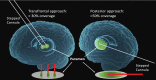
References
-
- Paul G, Sullivan AM (2019) Trophic factors for Parkinson’s disease: Where are we and where do we go from here? Eur J Neurosci 49, 440–452. - PubMed
-
- Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386, 896–912. - PubMed
-
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132. - PubMed
-
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER Jr., Lozano AM, Penn RD, Simpson RK Jr., Stacy M, Wooten GF, ICV GDNF Study Group. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60, 69–73. - PubMed
-
- Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM Jr., Milbrandt J (1996) Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature 384, 467–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

