Insight Into TLR4-Mediated Immunomodulation in Normal Pregnancy and Related Disorders
- PMID: 32508811
- PMCID: PMC7248557
- DOI: 10.3389/fimmu.2020.00807
Insight Into TLR4-Mediated Immunomodulation in Normal Pregnancy and Related Disorders
Abstract
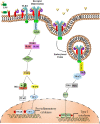
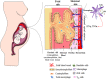
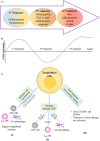
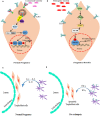
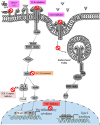
Unlike organ transplants where an immunosuppressive environment is required, a successful pregnancy involves an extremely robust, dynamic, and responsive maternal immune system to maintain the development of the fetus. A specific set of hormones and cytokines are associated with a particular stage of pregnancy. Any disturbance that alters this fine balance could compromise the development and function of the placenta. Although there are numerous underlying causes of pregnancy-related complications, untimely activation of Toll-like receptors (TLR), primarily TLR4, by intrauterine microbes poses the greatest risk. TLR4 is an important Pattern Recognition Receptor (PRR), which activates both innate and adaptive immune cells. TLR4 activation by LPS or DAMPs leads to the production of pro-inflammatory cytokines via the MyD88 dependent or independent pathway. Immune cells modulate the materno-fetal interface by TLR4-mediated cytokine production, which changes at different stages of pregnancy. In most pregnancy disorders, such as PTB, PE, or placental malaria, the TLR4 expression is upregulated in immune cells or in maternal derived cells, leading to the aberrant production of pro-inflammatory cytokines at the materno-fetal interface. Lack of functional TLR4 in mice has reduced the pro-inflammatory responses, leading to an improved pregnancy, which further strengthens the fact that abnormal TLR4 activation creates a hostile environment for the developing fetus. A recent study proposed that endothelial and perivascular stromal cells should interact with each other in order to maintain a homeostatic balance during TLR4-mediated inflammation. It has been reported that depleting immune cells or supplying anti-inflammatory cytokines can prevent PTB, PE, or fetal death. Blocking TLR4 signaling or its downstream molecule by inhibitors or antagonists has proven to improve pregnancy-related complications to some extent in clinical and animal models. To date, there has been a lack of knowledge regarding whether TLR4 accessories such as CD14 and MD-2 are important in pregnancy and whether these accessory molecules could be promising drug targets for combinatorial treatment of various pregnancy disorders. This review mainly focuses on the activation of TLR4 during pregnancy, its immunomodulatory functions, and the upcoming advancement in this field regarding the improvement of pregnancy-related issues by various therapeutic approaches.
Keywords: TLR4; innate immunity; pregnancy; preterm birth; pro-inflammatory.
Copyright © 2020 Firmal, Shah and Chattopadhyay.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

