Enhanced Pro-apoptotic Effects of Fe(II)-Modified IVIG on Human Neutrophils
- PMID: 32508840
- PMCID: PMC7248553
- DOI: 10.3389/fimmu.2020.00973
Enhanced Pro-apoptotic Effects of Fe(II)-Modified IVIG on Human Neutrophils
Abstract
Mild modification of intravenous immunoglobulin (IVIG) has been reported to result in enhanced polyspecificity and leveraged therapeutic effects in animal models of inflammation. Here, we observed that IVIG modification by ferrous ions, heme or low pH exposure, shifted the repertoires of specificities in different directions. Ferrous ions exposed Fe(II)-IVIG, but not heme or low pH exposed IVIG, showed increased pro-apoptotic effects on neutrophil granulocytes that relied on a FAS-dependent mechanism. These effects were also observed in human neutrophils primed by inflammatory mediators or rheumatoid arthritis joint fluid in vitro, or patient neutrophils ex vivo from acute Crohn's disease. These observations indicate that IVIG-mediated effects on cells can be enhanced by IVIG modification, yet specific modification conditions may be required to target specific molecular pathways and eventually to enhance the therapeutic potential.
Keywords: FAS; cell death; inflammation; modified IVIG; neutrophil.
Copyright © 2020 Graeter, Schneider, Verschoor, von Däniken, Seibold, Yawalkar, Villiger, Dimitrov, Smith, Cummings, Simon, Vassilev and von Gunten.
Figures
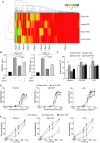
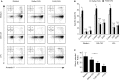
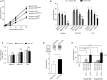
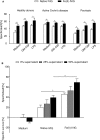
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

