Influence of Prolonged Dental Bleaching on the Adhesive Bond Strength to Enamel Surfaces
- PMID: 32508923
- PMCID: PMC7244969
- DOI: 10.1155/2020/2609359
Influence of Prolonged Dental Bleaching on the Adhesive Bond Strength to Enamel Surfaces
Abstract
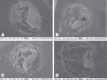
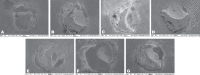
The objective of this in vitro study was to assess the influence of prolonged bleaching pre- and postrestoration on the bond strength (microshear) to enamel using 4% hydrogen peroxide (PH4). In the postrestorative bleached specimens evaluation, the composite cylinders were assembled after bleaching, while in the prebleached specimens, the cylinders were assembled before. Therefore, in the postbleached specimens, 60 bovine teeth were randomly assigned as follows: G1: control; G2: 14 days bleaching before bond strength (BS) testing; G3: 21 days; and G4: 28 days. In prebleached specimens, 180 bovine teeth were randomly assigned as follows: G1: control; G5: 14 days bleaching, storage in artificial saliva (AS) for 24 h before BS testing; G6: 14 days beaching, AS storage for 7 days before BS testing; G7: 21 days bleaching, AS storage for 24 h before BS testing; G8: 21 days bleaching, AS storage for 7 days before BS testing; G9: 28 days bleaching, AS storage for 24 hours before BS testing; and G10 : 28 days bleaching, AS storage for 7 days before BS testing. The results were submitted to ANOVA one-way (postrestoration bleaching) and two-way (prerestoration bleaching) and Tukey's post hoc test (p ≤ 0.05). In the postrestoration bleaching, no statistical difference between times was found. However, when bleached groups were compared to the control (G1), an expressive difference was detected (p ≤ 0.0001). For prerestoration bleaching, all experimental groups were statistically different from G1 (p ≤ 0.05), except G6 (p ≥ 0.01), and for G5 and G6, statistical differences were found (p ≤ 0.01). There were no significant differences between G7 and G8 and between G9 and G10, regardless of the AS storage times (p ≥ 0.05). It was concluded that prolonged bleaching with PH4 decreased adhesion resistance regardless of the moment of the bleaching (post- and prerestoration bleaching).
Copyright © 2020 Juliana C. P. Baia et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
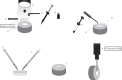

Similar articles
-
Influence of Prolonged Bleaching with 4% Hydrogen Peroxide Containing Calcium and Different Storage Times on the Bond Strength to Enamel.J Contemp Dent Pract. 2019 Feb 1;20(2):216-220. J Contemp Dent Pract. 2019. PMID: 31058638
-
Evaluation of the bond strength between a composite resin and enamel submitted to bleaching treatment and etched with Er:YAG laser.Photomed Laser Surg. 2011 Feb;29(2):91-5. doi: 10.1089/pho.2009.2728. Epub 2011 Jan 10. Photomed Laser Surg. 2011. PMID: 21219243
-
Does the Elapsed Time from Bleaching and the Use of Sodium Ascorbate Influence the Bond Strength of Resin Cement to Bleached Enamel?Materials (Basel). 2023 Sep 21;16(18):6328. doi: 10.3390/ma16186328. Materials (Basel). 2023. PMID: 37763605 Free PMC article.
-
The effect of in-office in combination with intracoronal bleaching on enamel and dentin bond strength and dentin morphology.J Contemp Dent Pract. 2008 Jul 1;9(5):17-24. J Contemp Dent Pract. 2008. PMID: 18633465
-
Effects of green tea application time on bond strength after enamel bleaching.Braz Dent J. 2014 Sep-Oct;25(5):399-403. doi: 10.1590/0103-6440201300015. Braz Dent J. 2014. PMID: 25517774
Cited by
-
Comparison of various methods of restoring adhesion to recently bleached enamel.BMC Oral Health. 2024 Aug 14;24(1):942. doi: 10.1186/s12903-024-04656-1. BMC Oral Health. 2024. PMID: 39143460 Free PMC article.
-
From Microstructure to Shade Shift: Confocal and Spectrophotometric Evaluation of Peroxide-Induced Dental Bleaching.J Clin Med. 2025 Jul 1;14(13):4642. doi: 10.3390/jcm14134642. J Clin Med. 2025. PMID: 40649016 Free PMC article.
-
Does the whitening dentifrice containing activated charcoal interfere with the properties of dental enamel? Microhardness, surface roughness and colorimetry analyzes.J Clin Exp Dent. 2024 Mar 1;16(3):e243-e249. doi: 10.4317/jced.60785. eCollection 2024 Mar. J Clin Exp Dent. 2024. PMID: 38600939 Free PMC article.
-
Assessing Tensile Bond Strength Between Denture Teeth and Nano-Zirconia Impregnated PMMA Denture Base.Int J Nanomedicine. 2020 Dec 1;15:9611-9625. doi: 10.2147/IJN.S273541. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33293810 Free PMC article.
-
Effect of enamel-surface modifications on shear bond strength using different adhesive materials.BMC Oral Health. 2022 Jun 7;22(1):224. doi: 10.1186/s12903-022-02254-7. BMC Oral Health. 2022. PMID: 35672818 Free PMC article.
References
-
- Swift E. J., Perdigao J. Effects of Bleaching on teeth and restorations. Compendium Continuing Education in Dentistry. 1998;19:815–820. - PubMed
-
- Breschi L., Cadenaro M., Antoniolli F., et al. Extent of polymerization of dental bonding systems on bleached enamel. The American Journal of Dentistry. 2007;20:275–280. - PubMed
-
- Lima A. F., Fonseca F. M., Cavalcanti A. N., et al. Effect of the diffusion of bleaching agents through enamel on dentin bonding at different depths. The American Journal of Dentistry. 2010;23:113–115. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

