Protective Effects of Biscoclaurine Alkaloids on Leukopenia Induced by 60Co- γ Radiation
- PMID: 32508944
- PMCID: PMC7251465
- DOI: 10.1155/2020/2162915
Protective Effects of Biscoclaurine Alkaloids on Leukopenia Induced by 60Co- γ Radiation
Abstract
Objective: Leukopenia, a common complication of tumor chemoradiotherapy, contributes serious damage to the hematopoietic, gastrointestinal, and immune systems of the body and can cause delay, discontinuation, or even failure to tumor treatment, thereby greatly threatening human health. The present study aims to investigate the protective effects of biscoclaurine alkaloids (BA) on leukopenia.
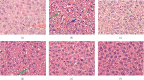
Methods: This study was conducted on 60 Kunming mice, which were randomly divided into six groups containing 10 animals each. A hematology analyzer was used to count white blood cells (WBC) in the peripheral blood cell. Mice serum was collected, and the granulocyte-macrophage colony-stimulating factor, vascular cell adhesion molecule 1 (VCAM-1), and interferon-γ (IFN-γ) were detected by enzyme-linked immunosorbent assays. Pathological changes were detected through hematoxylin and eosin staining in the liver and spleen of mice. The spleen and liver ultrastructures were observed via electron microscopy.
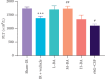
Results: Results showed that BA ameliorated WBC, PLT reduction in the peripheral blood and significantly increased the levels of IFN-γ and VCAM-1 in mice serum. BA reduced ionizing radiation-induced injuries to spleen, mitigated the reduction of superoxide dismutase (SOD), and significantly decreased the malonaldehyde (MDA) and xanthine oxidase (XOD) levels in the liver.
Conclusion: BA enhanced the immune and hematopoietic functions and ameliorated the oxidative stress induced by 60Co-γ radiation, revealing its therapeutic potential both as a radioprotector and as a radiation mitigator for leukopenia induced by 60Co-γ radiation.
Copyright © 2020 Min Wang et al.
Conflict of interest statement
The authors declare no conflicts of interest regarding the publication of this paper.
Figures





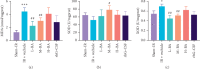

References
-
- Parente J. Diagnostics for white blood cell abnormalities. Physician Assistant Clinics. 2019;4(3):625–635. doi: 10.1016/j.cpha.2019.02.010. - DOI
-
- Singh V. K., Seed T. M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. International Journal of Radiation Biology. 2017;93(9):851–869. doi: 10.1080/09553002.2017.1332438. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

