The G2-to-M transition from a phosphatase perspective: a new vision of the meiotic division
- PMID: 32508972
- PMCID: PMC7249327
- DOI: 10.1186/s13008-020-00065-2
The G2-to-M transition from a phosphatase perspective: a new vision of the meiotic division
Abstract
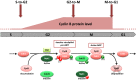
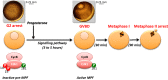
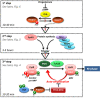
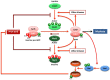
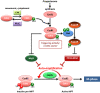
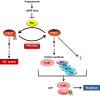
Cell division is orchestrated by the phosphorylation and dephosphorylation of thousands of proteins. These post-translational modifications underlie the molecular cascades converging to the activation of the universal mitotic kinase, Cdk1, and entry into cell division. They also govern the structural events that sustain the mechanics of cell division. While the role of protein kinases in mitosis has been well documented by decades of investigations, little was known regarding the control of protein phosphatases until the recent years. However, the regulation of phosphatase activities is as essential as kinases in controlling the activation of Cdk1 to enter M-phase. The regulation and the function of phosphatases result from post-translational modifications but also from the combinatorial association between conserved catalytic subunits and regulatory subunits that drive their substrate specificity, their cellular localization and their activity. It now appears that sequential dephosphorylations orchestrated by a network of phosphatase activities trigger Cdk1 activation and then order the structural events necessary for the timely execution of cell division. This review discusses a series of recent works describing the important roles played by protein phosphatases for the proper regulation of meiotic division. Many breakthroughs in the field of cell cycle research came from studies on oocyte meiotic divisions. Indeed, the meiotic division shares most of the molecular regulators with mitosis. The natural arrests of oocytes in G2 and in M-phase, the giant size of these cells, the variety of model species allowing either biochemical or imaging as well as genetics approaches explain why the process of meiosis has served as an historical model to decipher signalling pathways involved in the G2-to-M transition. The review especially highlights how the phosphatase PP2A-B55δ critically orchestrates the timing of meiosis resumption in amphibian oocytes. By opposing the kinase PKA, PP2A-B55δ controls the release of the G2 arrest through the dephosphorylation of their substrate, Arpp19. Few hours later, the inhibition of PP2A-B55δ by Arpp19 releases its opposing kinase, Cdk1, and triggers M-phase. In coordination with a variety of phosphatases and kinases, the PP2A-B55δ/Arpp19 duo therefore emerges as the key effector of the G2-to-M transition.
Keywords: Cdk1; Cell division; Meiotic division; Oocyte; PKA; PP2A; Protein kinases; Protein phosphatases; Protein phosphorylation.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
References
-
- Hunt T. Maturation promoting factor, cyclin and the control of M-phase. Curr Opin Cell Biol. 1989;1(2):268–274. - PubMed
-
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79(4):563–571. - PubMed
-
- Solomon M, Glotzer M, Lee T, Philippe M, Kirschner M. Cyclin activation of p34cdc2. Cell. 1990;63(5):1013–1024. - PubMed
-
- Karlsson-Rosenthal C, Millar JB. Cdc25: mechanisms of checkpoint inhibition and recovery. Trends Cell Biol. 2006;16(6):285–292. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

