Fluorinated phenylalanines: synthesis and pharmaceutical applications
- PMID: 32509033
- PMCID: PMC7237815
- DOI: 10.3762/bjoc.16.91
Fluorinated phenylalanines: synthesis and pharmaceutical applications
Abstract
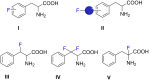
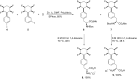
Recent advances in the chemistry of peptides containing fluorinated phenylalanines (Phe) represents a hot topic in drug research over the last few decades. ᴅ- or ʟ-fluorinated phenylalanines have had considerable industrial and pharmaceutical applications and they have been expanded also to play an important role as potential enzyme inhibitors as well as therapeutic agents and topography imaging of tumor ecosystems using PET. Incorporation of fluorinated aromatic amino acids into proteins increases their catabolic stability especially in therapeutic proteins and peptide-based vaccines. This review seeks to summarize the different synthetic approaches in the literature to prepare ᴅ- or ʟ-fluorinated phenylalanines and their pharmaceutical applications with a focus on published synthetic methods that introduce fluorine into the phenyl, the β-carbon or the α-carbon of ᴅ-or ʟ-phenylalanines.
Keywords: PET; fluorinated phenylalanines; pharmaceuticals application; α-fluorophenylalanine; β- and β,β-difluorophenylalanine.
Copyright © 2020, Awad and Ayoup; licensee Beilstein-Institut.
Figures
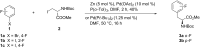
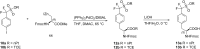
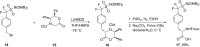

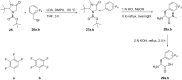

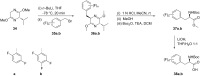
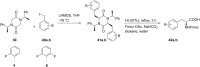

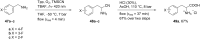
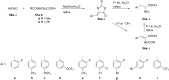
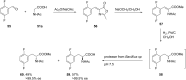


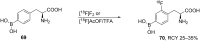

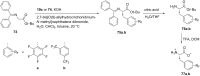
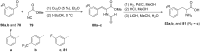
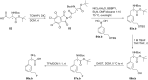

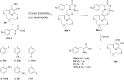
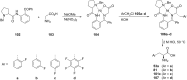

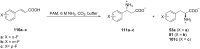

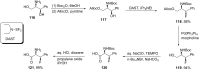

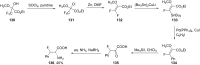

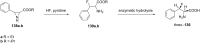
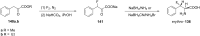




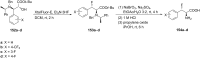
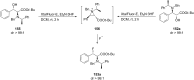

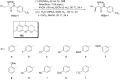
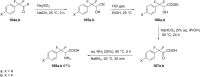


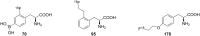
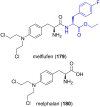
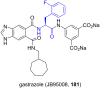

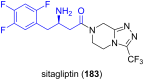
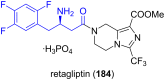

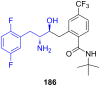


References
-
- Remete A M, Nonn M, Fustero S, Fülöp F, Kiss L. Tetrahedron. 2018;74:6367–6418. doi: 10.1016/j.tet.2018.09.021. - DOI
Publication types
LinkOut - more resources
Full Text Sources
