Automated Electrochemical Glucose Biosensor Platform as an Efficient Tool Toward On-Line Fermentation Monitoring: Novel Application Approaches and Insights
- PMID: 32509744
- PMCID: PMC7253623
- DOI: 10.3389/fbioe.2020.00436
Automated Electrochemical Glucose Biosensor Platform as an Efficient Tool Toward On-Line Fermentation Monitoring: Novel Application Approaches and Insights
Abstract
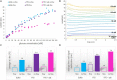
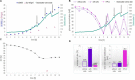
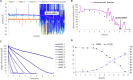
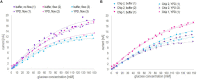
Monitoring and control of fermentation processes remain a crucial challenge for both laboratory and industrial-scale experiments. Reliable identification and quantification of the key process parameters in on-line mode allow operation of the fermentation at optimal reactor efficiency, maximizing productivity while minimizing waste. However, state-of-the-art fermentation on-line monitoring is still limited to a number of standard measurements such as pH, temperature and dissolved oxygen, as well as off-gas analysis as an advanced possibility. Despite the availability of commercial biosensor-based platforms that have been established for continuous monitoring of glucose and various biological variables within healthcare, on-line glucose quantification in fermentation processes has not been implemented yet to a large degree. For the first time, this work presents a complete study of a commercial flow-through-cell with integrated electrochemical glucose biosensors (1st generation) applied in different media, and importantly, at- and on-line during a yeast fed-batch fermentation process. Remarkably, the glucose biosensor-based platform combined with the developed methodology was able to detect glucose concentrations up to 150 mM in the complex fermentation broth, on both cell-free and cell-containing samples, when not compromised by oxygen limitations. This is four to six-fold higher than previously described in the literature presenting the application of biosensors predominately toward cell-free fermentation samples. The automated biosensor platform allowed reliable glucose quantification in a significantly less resource and time (<5 min) consuming manner compared to conventional HPLC analysis with a refractive index (RI) detector performed as reference measurement. Moreover, the presented biosensor platform demonstrated outstanding mechanical stability in direct contact with the fermentation medium and accurate glucose quantification in the presence of various electroactive species. Coupled with the developed methodology it can be readily considered as a simple, robust, accurate and inexpensive tool for real-time glucose monitoring in fermentation processes.
Keywords: bioprocesses; flow-through-cell; glucose biosensor; on-line monitoring; yeast fermentation.
Copyright © 2020 Pontius, Semenova, Silina, Gernaey and Junicke.
Figures


References
-
- Ampelli C., Leonardi S. G., Genovese C., Lanzafame P., Perathoner S., Centi G., et al. (2015). Monitoring of glucose in fermentation processes by using Au / TiO 2 composites as novel modified electrodes. J. Appl. Electrochem. 45 943–951. 10.1007/s10800-015-0874-4 - DOI
-
- Brooks S. L., Ashby R. E., Turner A. P. F., Calder M. R., Clarke D. J. (1987). Development of an on-line glucose sensor for fermentation monitoring. Biosensors 3 45–56. 10.1016/0265-928X(87)80012-3 - DOI
-
- Chen C., Xie Q., Yang D., Xiao H., Fu Y., Tan Y., et al. (2013). Recent advances in electrochemical glucose biosensors. RSC Adv. 3 4473–4491. 10.1039/c2ra22351a - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

