A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs
- PMID: 32510043
- PMCID: PMC7264956
- DOI: 10.1016/j.eclinm.2020.100293
A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs
Abstract
Background: HPV testing is replacing cytology for cervical cancer screening because of greater sensitivity and superior reassurance following negative tests for the dozen HPV genotypes that cause cervical cancer. Management of women testing positive is unresolved. The need for identification of individual HPV genotypes for clinical use is debated. Also, it is unclear how long to observe persistent infections when precancer is not initially found.
Methods: In the longitudinal NCI-Kaiser Permanente Northern California Persistence and Progression (PaP) Study, we observed the clinical outcomes (clearance, progression to CIN3+, or persistence without progression) of 11,573 HPV-positive women aged 30-65 yielding 14,158 type-specific infections.
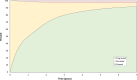
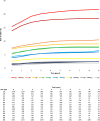
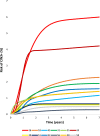
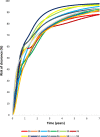
Findings: Risks of CIN3+ progression differed substantially by type, with HPV16 conveying uniquely elevated risk (26% of infections with seven-year CIN3+ risk of 22%). The other carcinogenic HPV types fell into 3 distinct seven-year CIN3+ risk groups: HPV18, 45 (13% of infections, risks >5%, with known elevated cancer risk); HPV31, 33, 35, 52, 58 (39%, risks >5%); and HPV39, 51, 56, 59, 68 (23%, risks <5%). In the absence of progression, HPV clearance rates were similar by type, with 80% of infections no longer detected within three years; persistence to seven years without progression was uncommon. The predictive value of abnormal cytology was most evident for prevalent CIN3+, but less evident in follow-up. A woman's age did not modify risk; rather it was the duration of persistence that was important.
Interpretation: HPV type and persistence are the major predictors of progression to CIN3+; at a minimum, distinguishing HPV16 is clinically important. Dividing the other HPV types into three risk-groups is worth considering.
Keywords: AGC, Atypical glandular cells; AIS, Adenocarcinoma in-situ; ASC-H+, Atypical squamous cells - cannot exclude HSIL; ASC-US, Atypical squamous cells of undetermined significance; BD, Becton Dickinson; CIN, Cervical intraepithelial neoplasia; HC2, Hybrid Capture 2; HPV genotype; HPV outcome, Clearance; HPV, human papillomavirus; KPNC, Kaiser Permanente Northern California; LSIL, Low-grade squamous intraepithelial lesion; NCI, National Cancer Institute; NILM, Negative for intraepithelial lesion or malignancy; PCR, Polymerase chain reaction; PaP, Persistence and Progression; Persistence; Progression; STM, Specimen transport medium.
© 2020 Published by Elsevier Ltd.
Conflict of interest statement
The following disclosure statements were reported: Dr. Demarco, Dr. Gage, Dr. Schiffman, and Dr. Wentzensen report that the NCI has received masked HPV and cytology test results at no cost from Roche Molecular Systems, BD Diagnostics, and Qiagen for independent evaluations of these technologies. Dr. Raine-Bennett reports other contracts from National Cancer Institute, during the conduct of the study. Dr. Campos reports other salary support from 10.13039/100000054National Cancer Institute, during the conduct of the study, and personal fees from Basic Health International, outside the submitted work. Dr. Coutlee reports grants from Réseau FRSQ SIDA-MI, during the conduct of the study. Dr. Burk reports grants from NIH, during the conduct of the study. Dr. Castle reports discounted or free HPV tests and assays for research from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corporation. Dr. Hyun, Dr. Carter-Pokras, Dr. Cheung, Dr. Chen, Dr. Hammer, Dr. Kinney, Dr. Befano, Dr. Perkins, Dr. He, Dr. Dallal, Dr. Chen, Dr. Poitras, Dr. Lorey, and Dr. Mayrand have nothing to disclose.
Figures

References
-
- Huh W.K., Ault K.A., Chelmow D. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

