HIGH-FREQUENCY failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal NON-ADHERENCE
- PMID: 32510047
- PMCID: PMC7264978
- DOI: 10.1016/j.eclinm.2020.100344
HIGH-FREQUENCY failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal NON-ADHERENCE
Abstract
Background: Early combination antiretroviral therapy (cART) reduces the size of the viral reservoir in paediatric and adult HIV infection. Very early-treated children may have higher cure/remission potential.
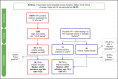
Methods: In an observational study of 151 in utero (IU)-infected infants in KwaZulu-Natal, South Africa, whose treatment adhered strictly to national guidelines, 76 infants diagnosed via point-of-care (PoC) testing initiated cART at a median of 26 h (IQR 18-38) and 75 infants diagnosed via standard-of-care (SoC) laboratory-based testing initiated cART at 10 days (IQR 8-13). We analysed mortality, time to suppression of viraemia, and maintenance of aviraemia over the first 2 years of life.
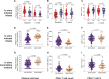
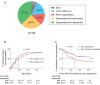
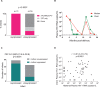
Findings: Baseline plasma viral loads were low (median 8000 copies per mL), with 12% of infants having undetectable viraemia pre-cART initiation. However, barely one-third (37%) of children achieved suppression of viraemia by 6 months that was maintained to >12 months. 24% had died or were lost to follow up by 6 months. Infant mortality was 9.3%. The high-frequency virological failure in IU-infected infants was associated not with transmitted or acquired drug-resistant mutations but with cART non-adherence (plasma cART undetectable/subtherapeutic, p<0.0001) and with concurrent maternal cART failure (OR 15.0, 95%CI 5.6-39.6; p<0.0001). High-frequency virological failure was observed in PoC- and SoC-tested groups of children.
Interpretation: The success of early infant testing and cART initiation strategies is severely limited by subsequent cART non-adherence in HIV-infected children. Although there are practical challenges to administering paediatric cART formulations, these are overcome by mothers who themselves are cART-adherent. These findings point to the ongoing obligation to address the unmet needs of the mothers. Eliminating the particular barriers preventing adequate treatment for these vulnerable women and infants need to be prioritised in order to achieve durable suppression of viraemia on cART, let alone HIV cure/remission, in HIV-infected children.
Funding: Wellcome Trust, National Institutes of Health.
Keywords: Hiv; Hiv cure; Mother-to-child transmission; Paediatric hiv.
© 2020 The Author(s).
Conflict of interest statement
Dr. Martinez-Picado reports institutional grants and educational/consultancy fees outside the submitted work from AbiVax, Astra-Zeneca, Gilead Sciences, Grifols, Janssen, Merck and ViiV Healthcare. Dr. Millar reports personal fees from Cepheid, outside the submitted work.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

