Carbon-fluorine bond cleavage mediated by metalloenzymes
- PMID: 32510080
- PMCID: PMC7375919
- DOI: 10.1039/c9cs00740g
Carbon-fluorine bond cleavage mediated by metalloenzymes
Abstract
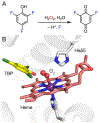
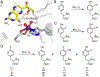
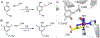
Fluorochemicals are a widely distributed class of compounds and have been utilized across a wide range of industries for decades. Given the environmental toxicity and adverse health threats of some fluorochemicals, the development of new methods for their decomposition is significant to public health. However, the carbon-fluorine (C-F) bond is among the most chemically robust bonds; consequently, the degradation of fluorinated hydrocarbons is exceptionally difficult. Here, metalloenzymes that catalyze the cleavage of this chemically challenging bond are reviewed. These enzymes include histidine-ligated heme-dependent dehaloperoxidase and tyrosine hydroxylase, thiolate-ligated heme-dependent cytochrome P450, and four nonheme oxygenases, namely, tetrahydrobiopterin-dependent aromatic amino acid hydroxylase, 2-oxoglutarate-dependent hydroxylase, Rieske dioxygenase, and thiol dioxygenase. While much of the literature regarding the aforementioned enzymes highlights their ability to catalyze C-H bond activation and functionalization, in many cases, the C-F bond cleavage has been shown to occur on fluorinated substrates. A copper-dependent laccase-mediated system representing an unnatural radical defluorination approach is also described. Detailed discussions on the structure-function relationships and catalytic mechanisms provide insights into biocatalytic defluorination, which may inspire drug design considerations and environmental remediation of halogenated contaminants.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
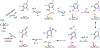
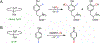
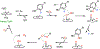
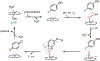

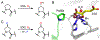
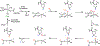
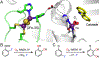
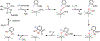
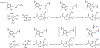

Similar articles
-
Cleavage of a carbon-fluorine bond by an engineered cysteine dioxygenase.Nat Chem Biol. 2018 Sep;14(9):853-860. doi: 10.1038/s41589-018-0085-5. Epub 2018 Jun 25. Nat Chem Biol. 2018. PMID: 29942080 Free PMC article.
-
Biocatalytic Carbon-Hydrogen and Carbon-Fluorine Bond Cleavage through Hydroxylation Promoted by a Histidyl-Ligated Heme Enzyme.ACS Catal. 2019 Jun 7;9(6):4764-4776. doi: 10.1021/acscatal.9b00231. Epub 2019 Apr 11. ACS Catal. 2019. PMID: 31355048 Free PMC article.
-
Confronting PFAS persistence: enzymes catalyzing C-F bond cleavage.Trends Biochem Sci. 2025 Jan;50(1):71-83. doi: 10.1016/j.tibs.2024.11.001. Epub 2024 Dec 5. Trends Biochem Sci. 2025. PMID: 39643519 Review.
-
Why Is the Biodegradation of Polyfluorinated Compounds So Rare?mSphere. 2021 Oct 27;6(5):e0072121. doi: 10.1128/mSphere.00721-21. Epub 2021 Oct 13. mSphere. 2021. PMID: 34643420 Free PMC article.
-
Tearing down to build up: Metalloenzymes in the biosynthesis lincomycin, hormaomycin and the pyrrolo [1,4]benzodiazepines.Biochim Biophys Acta. 2016 Jun;1864(6):724-737. doi: 10.1016/j.bbapap.2016.03.001. Epub 2016 Mar 7. Biochim Biophys Acta. 2016. PMID: 26963649 Review.
Cited by
-
Recent Advances in C-F Bond Cleavage Enabled by Visible Light Photoredox Catalysis.Molecules. 2021 Nov 22;26(22):7051. doi: 10.3390/molecules26227051. Molecules. 2021. PMID: 34834143 Free PMC article. Review.
-
Controlled monodefluorination and alkylation of C(sp3)-F bonds by lanthanide photocatalysts: importance of metal-ligand cooperativity.Chem Sci. 2022 Nov 8;13(47):14090-14100. doi: 10.1039/d2sc04192h. eCollection 2022 Dec 7. Chem Sci. 2022. PMID: 36540817 Free PMC article.
-
The link between ancient microbial fluoride resistance mechanisms and bioengineering organofluorine degradation or synthesis.Nat Commun. 2024 May 30;15(1):4593. doi: 10.1038/s41467-024-49018-1. Nat Commun. 2024. PMID: 38816380 Free PMC article. Review.
-
A novel catalytic heme cofactor in SfmD with a single thioether bond and a bis-His ligand set revealed by a de novo crystal structural and spectroscopic study.Chem Sci. 2021 Jan 22;12(11):3984-3998. doi: 10.1039/d0sc06369j. Chem Sci. 2021. PMID: 34163669 Free PMC article.
-
Study and design of amino acid-based radical enzymes using unnatural amino acids.RSC Chem Biol. 2023 May 18;4(6):431-446. doi: 10.1039/d2cb00250g. eCollection 2023 Jun 7. RSC Chem Biol. 2023. PMID: 37292061 Free PMC article.
References
-
- Berger R, Resnati G, Metrangolo P, Weber E and Hulliger J, Chem. Soc. Rev, 2011, 40, 3496–3508. - PubMed
-
- Gillis EP, Eastman KJ, Hill MD, Donnelly DJ and Meanwell NA, J. Med. Chem, 2015, 58, 8315–8359. - PubMed
-
- Shah P and Westwell AD, J. Enzyme Inhib. Med. Chem, 2007, 22, 527–540. - PubMed
-
- Hernandes MZ, Cavalcanti SM, Moreira DR, de Azevedo Junior WF and Leite AC, Curr. Drug Targets, 2010, 11, 303–314. - PubMed
-
- Analytics A, Global fluorochemical market: world market review by product type, by application, by end user industry (2019 Edition): opportunities and forecast (2019–2024), November, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

