Carbon-fluorine bond cleavage mediated by metalloenzymes
- PMID: 32510080
- PMCID: PMC7375919
- DOI: 10.1039/c9cs00740g
Carbon-fluorine bond cleavage mediated by metalloenzymes
Abstract
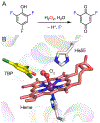
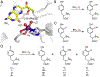
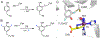
Fluorochemicals are a widely distributed class of compounds and have been utilized across a wide range of industries for decades. Given the environmental toxicity and adverse health threats of some fluorochemicals, the development of new methods for their decomposition is significant to public health. However, the carbon-fluorine (C-F) bond is among the most chemically robust bonds; consequently, the degradation of fluorinated hydrocarbons is exceptionally difficult. Here, metalloenzymes that catalyze the cleavage of this chemically challenging bond are reviewed. These enzymes include histidine-ligated heme-dependent dehaloperoxidase and tyrosine hydroxylase, thiolate-ligated heme-dependent cytochrome P450, and four nonheme oxygenases, namely, tetrahydrobiopterin-dependent aromatic amino acid hydroxylase, 2-oxoglutarate-dependent hydroxylase, Rieske dioxygenase, and thiol dioxygenase. While much of the literature regarding the aforementioned enzymes highlights their ability to catalyze C-H bond activation and functionalization, in many cases, the C-F bond cleavage has been shown to occur on fluorinated substrates. A copper-dependent laccase-mediated system representing an unnatural radical defluorination approach is also described. Detailed discussions on the structure-function relationships and catalytic mechanisms provide insights into biocatalytic defluorination, which may inspire drug design considerations and environmental remediation of halogenated contaminants.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
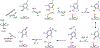
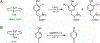
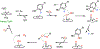
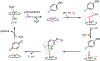

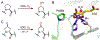
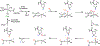
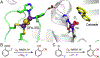
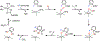
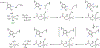

References
-
- Berger R, Resnati G, Metrangolo P, Weber E and Hulliger J, Chem. Soc. Rev, 2011, 40, 3496–3508. - PubMed
-
- Gillis EP, Eastman KJ, Hill MD, Donnelly DJ and Meanwell NA, J. Med. Chem, 2015, 58, 8315–8359. - PubMed
-
- Shah P and Westwell AD, J. Enzyme Inhib. Med. Chem, 2007, 22, 527–540. - PubMed
-
- Hernandes MZ, Cavalcanti SM, Moreira DR, de Azevedo Junior WF and Leite AC, Curr. Drug Targets, 2010, 11, 303–314. - PubMed
-
- Analytics A, Global fluorochemical market: world market review by product type, by application, by end user industry (2019 Edition): opportunities and forecast (2019–2024), November, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

