Overcoming Barriers: The Endothelium As a Linchpin of Coronavirus Disease 2019 Pathogenesis?
- PMID: 32510978
- PMCID: PMC7370857
- DOI: 10.1161/ATVBAHA.120.314558
Overcoming Barriers: The Endothelium As a Linchpin of Coronavirus Disease 2019 Pathogenesis?
Abstract
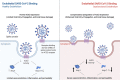
Objective: Coronavirus disease 2019 (COVID-19) is a global pandemic involving >5 500 000 cases worldwide as of May 26, 2020. The culprit is the severe acute respiratory syndrome coronavirus-2, which invades cells by binding to ACE2 (angiotensin-converting enzyme 2). While the majority of patients mount an appropriate antiviral response and recover at home, others progress to respiratory distress requiring hospital admission for supplemental oxygen. In severe cases, deterioration to acute respiratory distress syndrome necessitating mechanical ventilation, development of severe thrombotic events, or cardiac injury and dysfunction occurs. In this review, we highlight what is known to date about COVID-19 and cardiovascular risk, focusing in on the putative role of the endothelium in disease susceptibility and pathogenesis. Approach and Results: Cytokine-driven vascular leak in the lung alveolar-endothelial interface facilitates acute lung injury in the setting of viral infection. Given that the virus affects multiple organs, including the heart, it likely gains access into systemic circulation by infecting or passing from the respiratory epithelium to the endothelium for viral dissemination. Indeed, cardiovascular complications of COVID-19 are highly prevalent and include acute cardiac injury, myocarditis, and a hypercoagulable state, all of which may be influenced by altered endothelial function. Notably, the disease course is worse in individuals with preexisting comorbidities that involve endothelial dysfunction and may be linked to elevated ACE2 expression, such as diabetes mellitus, hypertension, and cardiovascular disease.
Conclusions: Rapidly emerging data on COVID-19, together with results from studies on severe acute respiratory syndrome coronavirus-1, are providing insight into how endothelial dysfunction may contribute to the pandemic that is paralyzing the globe. This may, in turn, inform the design of biomarkers predictive of disease course, as well as therapeutics targeting pathogenic endothelial responses.
Keywords: biomarkers; cardiac injury; coronavirus; endothelium; pandemic.
Figures

References
-
- World Health Organization. Novel Coronavirus (2019-nCoV)-SITUATION REPORT-1. 2020 https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed April 6, 2020.
-
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK, Franco-Paredes C, Henao-Martinez AF, et al. ; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19) Electronic address: https://www.lancovid.org. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 202034101623.doi: 10.1016/j.tmaid.2020.101623 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

