Efficacy of intravenous iron treatment for chemotherapy-induced anemia: A prospective Phase II pilot clinical trial in South Korea
- PMID: 32511251
- PMCID: PMC7279571
- DOI: 10.1371/journal.pmed.1003091
Efficacy of intravenous iron treatment for chemotherapy-induced anemia: A prospective Phase II pilot clinical trial in South Korea
Abstract
Background: Anemia is the most common and serious cancer-related complication. This study aimed to evaluate the efficacy of administration of ferric carboxymaltose without erythropoiesis-stimulating agents for treating anemia in cancer patients. Moreover, we identified the biomarkers of hemoglobin response to predict the need for iron therapy.
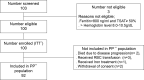
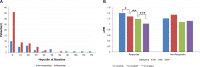
Methods and findings: We enrolled patients with solid cancers who were treated at a single institute (Samsung Medical Center, South Korea), from April 2015 to July 2017, in this prospective single-arm Phase II clinical trial. Patients received intravenous ferric carboxymaltose (1,000 mg) infusion on the first day (visit 1) of treatment. The primary end point was the number of hemoglobin responders, defined as patients with an increase in hemoglobin level ≥ 1.0 g/dL from the baseline, a hemoglobin level ≥ 11.0 g/dL, or both, within an 8-week observation period (week 3, 6, or 8). Secondary end points included changes in transferrin saturation and levels of soluble transferrin receptors, hepcidin, erythropoietin, interleukin-6, and C-reactive protein (CRP) at each visit. Of the 103 recruited patients, 92 were eligible for analysis. The mean patient age was 57.3 ± 12.5 years, and 54.3% of the patients were women. The most common diagnoses were breast cancer (n = 23, 25.1%), lung cancer (n = 21, 22.9%), gastrointestinal cancer (n = 20, 20.9%), and lymphoma (n = 16, 17.7%). A hemoglobin response was observed in 36 (39.1%), 53 (57.6%), and 61 (66.3%) patients in the third, fifth, and eighth weeks, respectively. The mean increase in hemoglobin levels from the baseline to the end of treatment was 1.77 ± 1.30 g/dL. Baseline values of hepcidin (p = 0.008), total iron binding capacity (p = 0.014), ferritin (p = 0.048), and CRP (p = 0.044) were significantly different between the responder and nonresponder groups. Multiple logistic regression analysis for baseline anemia-related biochemical variable significantly associated with the hemoglobin response showed that only baseline hepcidin level was a significant factor for hemoglobin response (odds ratio = 0.95, 95% confidence interval 0.90-1.0, p = 0.045). Hemoglobin responders had significantly lower hepcidin levels than nonresponders (mean [±standard deviation], 13.45 [±14.71] versus 35.22 [±40.470 ng/ml]; p = 0.007). However, our analysis had some limitations such as the different patient characteristics in the studies that were included, institutional differences in the measurement of hepcidin level, and missing data on long-term safety. Therefore, our findings need further validation.
Conclusions: Intravenous ferric carboxymaltose (1,000 mg) monotherapy increases hemoglobin levels without serious adverse events in patients with cancer. Hepcidin is a useful biomarker for predicting iron requirement in cancer patients.
Trial registration: Clinicaltrials.gov NCT02599012.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Effect of Intravenous Ferric Carboxymaltose on Hemoglobin Response Among Patients With Acute Isovolemic Anemia Following Gastrectomy: The FAIRY Randomized Clinical Trial.JAMA. 2017 May 23;317(20):2097-2104. doi: 10.1001/jama.2017.5703. JAMA. 2017. PMID: 28535237 Free PMC article. Clinical Trial.
-
Switching patients with non-dialysis chronic kidney disease from oral iron to intravenous ferric carboxymaltose: effects on erythropoiesis-stimulating agent requirements, costs, hemoglobin and iron status.PLoS One. 2015 Apr 30;10(4):e0125528. doi: 10.1371/journal.pone.0125528. eCollection 2015. PLoS One. 2015. PMID: 25928811 Free PMC article. Clinical Trial.
-
Intravenous iron alone resolves anemia in patients with functional iron deficiency and lymphoid malignancies undergoing chemotherapy.Med Oncol. 2014 Dec;31(12):302. doi: 10.1007/s12032-014-0302-3. Epub 2014 Nov 6. Med Oncol. 2014. PMID: 25373320 Free PMC article. Clinical Trial.
-
A meta-analysis of ferric carboxymaltose versus other intravenous iron preparations for the management of iron deficiency anemia during pregnancy.Rev Bras Ginecol Obstet. 2024 Mar 15;46:e-rbgo21. doi: 10.61622/rbgo/2024AO21. eCollection 2024. Rev Bras Ginecol Obstet. 2024. PMID: 38765534 Free PMC article. Review.
-
The Effect of Perioperative Intravenous Iron on Hemoglobin in Surgical Patients: A Meta-Analysis.J Surg Res. 2020 Feb;246:42-51. doi: 10.1016/j.jss.2019.08.023. Epub 2019 Sep 24. J Surg Res. 2020. PMID: 31561177
Cited by
-
Seven-Year Single-Center Experience of the Efficacy and Safety of Ferric Carboxymaltose in Cancer Patients with Iron-Deficiency Anemia.Curr Oncol. 2023 Nov 2;30(11):9689-9700. doi: 10.3390/curroncol30110703. Curr Oncol. 2023. PMID: 37999123 Free PMC article.
-
Experimental Drugs for Chemotherapy- and Cancer-Related Anemia.J Exp Pharmacol. 2021 Jun 24;13:593-611. doi: 10.2147/JEP.S262349. eCollection 2021. J Exp Pharmacol. 2021. PMID: 34194245 Free PMC article. Review.
-
Anaemia and pathologic complete response rate according to carboplatin dose in HER2+ breast cancer treated with neoadjuvant TCHP.Cancer Med. 2023 Jan;12(2):1409-1417. doi: 10.1002/cam4.5022. Epub 2022 Jul 15. Cancer Med. 2023. PMID: 35837812 Free PMC article.
-
Efficacy of ferric carboxymaltose or darbepoetin alfa for chemotherapy-induced anemia in patients with esophagogastric or pancreaticobiliary cancer: a retrospective comparative study.Ther Adv Med Oncol. 2024 Jul 31;16:17588359241265209. doi: 10.1177/17588359241265209. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39091605 Free PMC article.
-
Efficacy and safety of ferric carboxymaltose infusion in reducing anemia in patients receiving chemotherapy for nonmyeloid malignancies: A randomized, placebo-controlled study (IRON-CLAD).Am J Hematol. 2021 Dec 1;96(12):1639-1646. doi: 10.1002/ajh.26376. Epub 2021 Nov 19. Am J Hematol. 2021. PMID: 34653287 Free PMC article. Clinical Trial.
References
-
- Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004; 40(15):2293–306. 10.1016/j.ejca.2004.06.019 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

