Three-dimensional ultrastructure of Plasmodium falciparum throughout cytokinesis
- PMID: 32511279
- PMCID: PMC7302870
- DOI: 10.1371/journal.ppat.1008587
Three-dimensional ultrastructure of Plasmodium falciparum throughout cytokinesis
Abstract
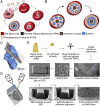
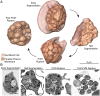
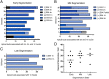
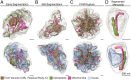
New techniques for obtaining electron microscopy data through the cell volume are being increasingly utilized to answer cell biologic questions. Here, we present a three-dimensional atlas of Plasmodium falciparum ultrastructure throughout parasite cell division. Multiple wild type schizonts at different stages of segmentation, or budding, were imaged and rendered, and the 3D structure of their organelles and daughter cells are shown. Our high-resolution volume electron microscopy both confirms previously described features in 3D and adds new layers to our understanding of Plasmodium nuclear division. Interestingly, we demonstrate asynchrony of the final nuclear division, a process that had previously been reported as synchronous. Use of volume electron microscopy techniques for biological imaging is gaining prominence, and there is much we can learn from applying them to answer questions about Plasmodium cell biology. We provide this resource to encourage readers to consider adding these techniques to their cell biology toolbox.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ. Manson’s Tropical Diseases E-Book: Elsevier Health Sciences; 2013.
-
- Read M, Sherwin T, Holloway SP, Gull K, Hyde JE. Microtubular organization visualized by immunofluorescence microscopy during erythrocytic schizogony in Plasmodium falciparum and investigation of post-translational modifications of parasite tubulin. Parasitology. 1993;106 (Pt 3):223–32. Epub 1993/04/01. 10.1017/s0031182000075041 . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

