This is a preprint.
Structural Similarity of SARS-CoV2 Mpro and HCV NS3/4A Proteases Suggests New Approaches for Identifying Existing Drugs Useful as COVID-19 Therapeutics
- PMID: 32511291
- PMCID: PMC7263768
- DOI: 10.26434/chemrxiv.12153615
Structural Similarity of SARS-CoV2 Mpro and HCV NS3/4A Proteases Suggests New Approaches for Identifying Existing Drugs Useful as COVID-19 Therapeutics
Update in
-
Hepatitis C virus drugs that inhibit SARS-CoV-2 papain-like protease synergize with remdesivir to suppress viral replication in cell culture.Cell Rep. 2021 May 18;35(7):109133. doi: 10.1016/j.celrep.2021.109133. Epub 2021 Apr 27. Cell Rep. 2021. PMID: 33984267 Free PMC article.
Abstract
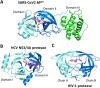
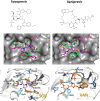
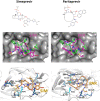
During the current COVID-19 pandemic more than 160,000 people have died worldwide as of mid-April 2020, and the global economy has been crippled. Effective control of the SARS-CoV2 virus that causes the COVID-19 pandemic requires both vaccines and antivirals. Antivirals are particularly crucial to treat infected people during the period of time that an effective vaccine is being developed and deployed. Because the development of specific antiviral drugs can take a considerable length of time, an important approach is to identify existing drugs already approved for use in humans which could be repurposed as COVID-19 therapeutics. Here we focus on antivirals directed against the SARS-CoV2 Mpro protease, which is required for virus replication. A structural similarity search showed that the Hepatitis C virus (HCV) NS3/4A protease has a striking three-dimensional structural similarity to the SARS-CoV2 Mpro protease, particularly in the arrangement of key active site residues. We used virtual docking predictions to assess the hypothesis that existing drugs already approved for human use or clinical testing that are directed at the HCV NS3/4A protease might fit well into the active-site cleft of the SARS-CoV2 protease (Mpro). AutoDock docking scores for 12 HCV protease inhibitors and 9 HIV-1 protease inhibitors were determined and compared to the docking scores for an α-ketoamide inhibitor of Mpro, which has recently been shown to inhibit SARS-CoV2 virus replication in cell culture. We identified eight HCV protease inhibitors that bound to the Mpro active site with higher docking scores than the α-ketoamide inhibitor, suggesting that these protease inhibitors may effectively bind to the Mpro active site. These results provide the rationale for us to test the identified HCV protease inhibitors as inhibitors of the SARS-CoV2 protease, and as inhibitors of SARS-CoV2 virus replication. Subsequently these repurposed drugs could be evaluated as COVID-19 therapeutics.
Keywords: COVID-19; Drug Discovery; Hepatitis C Virus (HCV) NS3/4A protease inhibitors; Human Immunodeficiency Virus 1 (HIV-1) protease inhibitors; Severe Acute Respiratory Syndrome (SARS); structural bioinformatics.
Conflict of interest statement
Conflict of Interest Statement: G.T.M. is a founder of Nexomics Biosciences, Inc.
Figures





References
-
- Gordon D. E., Jang G. M., Bouhaddou M., Xu J., Obernier K., O’meara M. J., Guo J. Z., Swaney D. L., Tummino T. A., Huettenhain R., et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. bioRxiv 2020. 10.1101/2020.03.22.002386 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
